Quality Systems Approach Overview
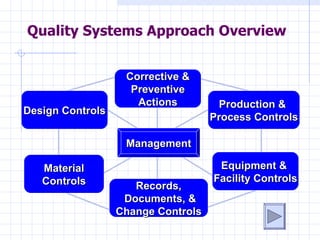
- 1. Quality Systems Approach Overview Corrective & Preventive Actions Production & Design Controls Process Controls Management Material Equipment & Controls Facility Controls Records, Documents, & Change Controls
- 2. Quality System Alignment and Integration Continuous Improvement Design Execute Monitor/Control Design – determine what is really important Execute – translate into service and manufacturing Monitor/Control – translate into quality assurance
- 4. Capability and Control Cycle Development History Integrated Validation Master Plan process product systems Begin with the End in Mind 2. Compliant Establish shared standards 3. Capable Process Flow Document and expectations 4. Robust 5. In Control 6. Continuously Improving Technology Transfer Qualification Document with FDA in Mind 2. Clear Execute and monitor with 3. Concise Validation process and product best business practices. 4. Correct 5. Complete Execute, Monitor and Control 6. Confident Technical Evaluations process and product Quality (GMP) Evaluations quality systems Change Control process and product Change Control quality systems Assess results against the standards and practices. Quality Management System
- 8. Scenario Worst Case Most Likely Case Best Case Decision
- 10. Responsibility of Highest Level of Management Establish Quality Policy Ensure that it is followed
- 11. Delegation by Management with Executive Responsibility Establishment of quality objectives Translation of objectives into methods and procedures Implementation of quality system
- 12. How does Management Assure an Effective Quality System? CAPA Management Audits Review
- 13. How to Demonstrate Compliance Procedures ... Verbal Communications Written records and documents
- 14. Establish [21 CFR 820.3(k)] Define Document Implement
- 15. Key Elements of a Quality Manual 1. Generation and maintenance of master production batch records. 2. Generation of routine batch records 3. Generation and maintenance of Standard Operating Procedures 4. Generation and maintenance of preventive maintenance procedures 5. Generation and maintenance of calibration procedures 6. Generation and maintenance of equipment logbooks 7. Generation and maintenance of cleaning procedures 8. Generation and maintenance of deviation/failure reports 9. Generation of rework procedures
- 16. Key Elements of a Quality Manual 1. Training programs and records for all employees 2. In-coming inspection program for raw materials 3. In-process analytical checks during processing 4. Inventory control 6. Validation of equipment/systems/processes 7. Cleaning validation 8. Analytical methods validation 9. Computer/controller validation 10. Validation change control 11. Revalidation program
- 17. Key Elements of a Quality Manual 1. Audit programs - internally and externally 2. Qualification of vendors 3. Quality Control testing (in-process and finished product testing) 4. Complaint handling program 5. Annual product reviews 6. Stability Program 7. Sample retention program 8. Documentation control/storage 9. Labeling and label control 10. Specification development 11. Generation and validation of analytical methods
- 18. Writing and Managing Standard Operating Procedures Controls Approvals Formatting Readability Change History Cross References
- 19. Developing Batch Records CFR 211.188 Prepared for each batch of drug product produced Include complete information relating to production and control of each batch (signed, dated and checked accurate reproduction of master production or control record, documentation of accomplished significant steps in manufacture, processing, packing or holding – dates, equipment and lines, specific identification of components and in-process material, ….)
- 20. Document Management Structure Segregation Documents in Review Documents in Approval Effective Documents Archived Documents Control
- 21. Complaint Management CFR 211.198 Mechanism Designated person in quality group Logged with unique number Sender Detail Sample Appropriate storage conditions Investigation methodology Response Reference book
- 22. Annual Product Review CFR 211.180 (e) FDA Requirement Annual Reports Summary of all findings Sent to FDA by product NDA anniversary date Review of batches Deviations Failures Out of Specifications Stability Profiles Visual verification Retained samples
- 23. Managing Regulatory Training Compliance CFR 211.25 Education, Training, and Experience Enable to perform assigned functions Particular operations performed Current Good Manufacturing Practice Written procedures related to assigned functions Continuing basis Sufficient frequency Documented Follow-up
- 24. How does Management Assure an Effective Quality System? CAPA Management Audits Review
- 25. (Your Organization’s Name) Regulatory Responsibilities Job Title/Employee Quality System 21 CFR Part 211 Subparts A B C D E F G H I J K
- 26. Management Production & Design Controls Process Controls Corrective & Preventive Actions Material Equipment & Controls Facility Controls Records, Documents, & Change Controls Controls
- 27. (Your Organization’s Name) Regulatory Responsibilities • What are the responsibilities? • Where are the gaps? • What are the risks? • What are the consequences? • What are the opportunities? • What are the rewards?
- 28. QUESTIONS
