Types of bonding in solids
•Download as PPTX, PDF•
58 likes•44,975 views
Report
Share
Report
Share
Recommended
Recommended
NANO106 is UCSD Department of NanoEngineering's core course on crystallography of materials taught by Prof Shyue Ping Ong. For more information, visit the course wiki at http://nano106.wikispaces.com.UCSD NANO106 - 03 - Lattice Directions and Planes, Reciprocal Lattice and Coo...

UCSD NANO106 - 03 - Lattice Directions and Planes, Reciprocal Lattice and Coo...University of California, San Diego
More Related Content
What's hot
NANO106 is UCSD Department of NanoEngineering's core course on crystallography of materials taught by Prof Shyue Ping Ong. For more information, visit the course wiki at http://nano106.wikispaces.com.UCSD NANO106 - 03 - Lattice Directions and Planes, Reciprocal Lattice and Coo...

UCSD NANO106 - 03 - Lattice Directions and Planes, Reciprocal Lattice and Coo...University of California, San Diego
What's hot (20)
Space lattice and crystal structure,miller indices PEC UNIVERSITY CHD

Space lattice and crystal structure,miller indices PEC UNIVERSITY CHD
UCSD NANO106 - 03 - Lattice Directions and Planes, Reciprocal Lattice and Coo...

UCSD NANO106 - 03 - Lattice Directions and Planes, Reciprocal Lattice and Coo...
Similar to Types of bonding in solids
The Indian Dental Academy is the Leader in continuing dental education , training dentists in all aspects of dentistry and offering a wide range of dental certified courses in different formats.
Indian dental academy provides dental crown & Bridge,rotary endodontics,fixed orthodontics,
Dental implants courses.for details pls visit www.indiandentalacademy.com ,or call
0091-9248678078
Structure of matter /certified fixed orthodontic courses by Indian dental aca...

Structure of matter /certified fixed orthodontic courses by Indian dental aca...Indian dental academy
Similar to Types of bonding in solids (20)
Structure of matter /certified fixed orthodontic courses by Indian dental aca...

Structure of matter /certified fixed orthodontic courses by Indian dental aca...
Recently uploaded
A Principled Technologies deployment guide
Conclusion
Deploying VMware Cloud Foundation 5.1 on next gen Dell PowerEdge servers brings together critical virtualization capabilities and high-performing hardware infrastructure. Relying on our hands-on experience, this deployment guide offers a comprehensive roadmap that can guide your organization through the seamless integration of advanced VMware cloud solutions with the performance and reliability of Dell PowerEdge servers. In addition to the deployment efficiency, the Cloud Foundation 5.1 and PowerEdge solution delivered strong performance while running a MySQL database workload. By leveraging VMware Cloud Foundation 5.1 and PowerEdge servers, you could help your organization embrace cloud computing with confidence, potentially unlocking a new level of agility, scalability, and efficiency in your data center operations.Deploy with confidence: VMware Cloud Foundation 5.1 on next gen Dell PowerEdg...

Deploy with confidence: VMware Cloud Foundation 5.1 on next gen Dell PowerEdg...Principled Technologies
💉💊+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHABI}}+971581248768
+971581248768 Mtp-Kit (500MG) Prices » Dubai [(+971581248768**)] Abortion Pills For Sale In Dubai, UAE, Mifepristone and Misoprostol Tablets Available In Dubai, UAE CONTACT DR.Maya Whatsapp +971581248768 We Have Abortion Pills / Cytotec Tablets /Mifegest Kit Available in Dubai, Sharjah, Abudhabi, Ajman, Alain, Fujairah, Ras Al Khaimah, Umm Al Quwain, UAE, Buy cytotec in Dubai +971581248768''''Abortion Pills near me DUBAI | ABU DHABI|UAE. Price of Misoprostol, Cytotec” +971581248768' Dr.DEEM ''BUY ABORTION PILLS MIFEGEST KIT, MISOPROTONE, CYTOTEC PILLS IN DUBAI, ABU DHABI,UAE'' Contact me now via What's App…… abortion Pills Cytotec also available Oman Qatar Doha Saudi Arabia Bahrain Above all, Cytotec Abortion Pills are Available In Dubai / UAE, you will be very happy to do abortion in Dubai we are providing cytotec 200mg abortion pill in Dubai, UAE. Medication abortion offers an alternative to Surgical Abortion for women in the early weeks of pregnancy. We only offer abortion pills from 1 week-6 Months. We then advise you to use surgery if its beyond 6 months. Our Abu Dhabi, Ajman, Al Ain, Dubai, Fujairah, Ras Al Khaimah (RAK), Sharjah, Umm Al Quwain (UAQ) United Arab Emirates Abortion Clinic provides the safest and most advanced techniques for providing non-surgical, medical and surgical abortion methods for early through late second trimester, including the Abortion By Pill Procedure (RU 486, Mifeprex, Mifepristone, early options French Abortion Pill), Tamoxifen, Methotrexate and Cytotec (Misoprostol). The Abu Dhabi, United Arab Emirates Abortion Clinic performs Same Day Abortion Procedure using medications that are taken on the first day of the office visit and will cause the abortion to occur generally within 4 to 6 hours (as early as 30 minutes) for patients who are 3 to 12 weeks pregnant. When Mifepristone and Misoprostol are used, 50% of patients complete in 4 to 6 hours; 75% to 80% in 12 hours; and 90% in 24 hours. We use a regimen that allows for completion without the need for surgery 99% of the time. All advanced second trimester and late term pregnancies at our Tampa clinic (17 to 24 weeks or greater) can be completed within 24 hours or less 99% of the time without the need surgery. The procedure is completed with minimal to no complications. Our Women's Health Center located in Abu Dhabi, United Arab Emirates, uses the latest medications for medical abortions (RU-486, Mifeprex, Mifegyne, Mifepristone, early options French abortion pill), Methotrexate and Cytotec (Misoprostol). The safety standards of our Abu Dhabi, United Arab Emirates Abortion Doctors remain unparalleled. They consistently maintain the lowest complication rates throughout the nation. Our Physicians and staff are always available to answer questions and care for women in one of the most difficult times in their lives. The decision to have an abortion at the Abortion Cl+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...

+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...?#DUbAI#??##{{(☎️+971_581248768%)**%*]'#abortion pills for sale in dubai@
Recently uploaded (20)
Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood

Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood
Cloud Frontiers: A Deep Dive into Serverless Spatial Data and FME

Cloud Frontiers: A Deep Dive into Serverless Spatial Data and FME
Why Teams call analytics are critical to your entire business

Why Teams call analytics are critical to your entire business
Mastering MySQL Database Architecture: Deep Dive into MySQL Shell and MySQL R...

Mastering MySQL Database Architecture: Deep Dive into MySQL Shell and MySQL R...
Connector Corner: Accelerate revenue generation using UiPath API-centric busi...

Connector Corner: Accelerate revenue generation using UiPath API-centric busi...
Deploy with confidence: VMware Cloud Foundation 5.1 on next gen Dell PowerEdg...

Deploy with confidence: VMware Cloud Foundation 5.1 on next gen Dell PowerEdg...
HTML Injection Attacks: Impact and Mitigation Strategies

HTML Injection Attacks: Impact and Mitigation Strategies
Exploring the Future Potential of AI-Enabled Smartphone Processors

Exploring the Future Potential of AI-Enabled Smartphone Processors
Apidays New York 2024 - Scaling API-first by Ian Reasor and Radu Cotescu, Adobe

Apidays New York 2024 - Scaling API-first by Ian Reasor and Radu Cotescu, Adobe
Tata AIG General Insurance Company - Insurer Innovation Award 2024

Tata AIG General Insurance Company - Insurer Innovation Award 2024
Top 10 Most Downloaded Games on Play Store in 2024

Top 10 Most Downloaded Games on Play Store in 2024
Powerful Google developer tools for immediate impact! (2023-24 C)

Powerful Google developer tools for immediate impact! (2023-24 C)
Bajaj Allianz Life Insurance Company - Insurer Innovation Award 2024

Bajaj Allianz Life Insurance Company - Insurer Innovation Award 2024
The 7 Things I Know About Cyber Security After 25 Years | April 2024

The 7 Things I Know About Cyber Security After 25 Years | April 2024
Bajaj Allianz Life Insurance Company - Insurer Innovation Award 2024

Bajaj Allianz Life Insurance Company - Insurer Innovation Award 2024
+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...

+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...
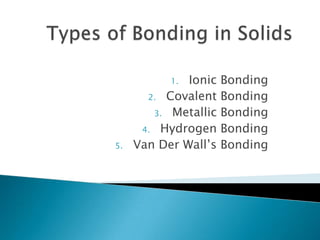
Types of bonding in solids
- 1. Ionic 2. Covalent 3. Metallic 4. Hydrogen Van Der Wall’s 1. 5. Bonding Bonding Bonding Bonding Bonding
- 2. An ionic bonding is the Attractive Force existing between positive ion and a negative ion when they are brought into close proximity or surrounding. They are formed when atoms of different elements lose or gain their electrons in order to achieve stabilized outermost electronic configuration. Ionic Bonding in NaCl
- 3. Ionic solids are rigid, unidirectional and crystalline in nature. They have high melting and boiling points. Ionic solids are good insulators of electricity in their solid state and good conductor of electricity in their molten state. Ionic solids are soluble in water and slightly soluble in organic solvents.
- 4. A covalent bond is formed, when two or more electrons of an atom, in its outermost energy level, are shared by other atoms. e.g.-Chlorine molecule. In this bonding a stable arrangement is achieved by sharing of electrons rather than transfer of electrons. Sometimes a covalent bond is also formed when two atoms of different non-metals share one or more pair of electrons in their outermost energy level. e.g.- Water molecule Bonding between two atoms of same element Bonding between two different nonmetals
- 5. Covalent compounds are bad conductors of electricity. Covalent compounds are having low melting and boiling points. Insoluble- in water Soluble- in organic solvents like Benzene
- 6. It has been observed that in a metal atoms, the electrons in their outermost energy levels are loosely held by their nucleii. Thus a metal may be considered as a cluster of positive ions surrounded by a large number of free electrons, forming electron cloud. e.g.- all metals
- 7. High thermal and electrical conductivity Low melting and boiling point temperature Have a bright lustre Metallic solids are malleable and ductile
- 8. Covalently bonded atoms often produce an Electric dipole configuration with hydrogen atom as the positive end of the dipole. If bonds arise as a result of electrostatic attraction between atoms, it is known as hydrogen bonding.
- 9. The hydrogen bonds are directional Relatively strong bonding These solids have low melting point No valence electrons hence good insulators Soluble in both polar and non-polar solvents They are transparent to light e.g. – water molecule, ammonic molecules
- 10. Weak and temporary bonds between molecules of the same substance are known as Van der Walls bonding. Types of Van der walls forces 1) dipole-dipole 2) dipole-induced dipole 3) dispersion
- 11. Crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting longrange order and symmetry. Patterns are located upon the points of a lattice, which is an array of points repeating periodically in three dimensions. The points can be thought of as forming identical tiny boxes, called unit cells, that fill the space of the lattice. The lengths of the edges of a unit cell and the angles between them are called the lattice parameters.
- 13. Bond strength is the degree to which each atom joined to another in a chemical bond contributes to the valency of this other atom. As the number of bonds between two atoms increases, the bond grows shorter and stronger. Types of Bonds Single bond Double bond Triple Bond Bond Energy – Amount of energy required to break one mole of bonds. Bond strength depends upon no. of bonds present in molecule. for e.g.- C=C is stronger than C-C.
- 14. Melting Point: The temperature at which a solid becomes liquid. If heat is applied to a solid, its temperature rises until the melting point is reached, when heat energy is then absorbed to form liquid from the solid is then absorbed to form liquid from the solid. Temperature continues to rise once the melting is complete Variation in melting point in Periodic table
