Chpt1 11
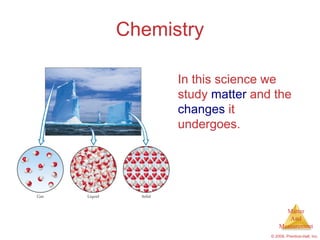
- 1. Chemistry In this science we study matter and the changes it undergoes. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 2. Matter We define matter as anything that has mass and takes up space. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 3. Matter • Atoms are the building blocks of matter. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 4. Matter • Atoms are the building blocks of matter. • Each element is made of the same kind of atom. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 5. Matter • Atoms are the building blocks of matter. • Each element is made of the same kind of atom. • A compound is made of two or more different kinds of elements. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 6. States of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 7. Classification of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 8. Classification of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 9. Classification of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 10. Classification of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 11. Classification of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 12. Classification of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 13. Classification of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 14. Classification of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 15. Classification of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 16. Classification of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 17. Properties and Changes of Matter Matter And Measurement © 2009, Prentice-Hall, Inc.
- 18. Types of Properties • Physical Properties… – Can be observed without changing a substance into another substance. • Boiling point, density, mass, volume, etc. • Chemical Properties… – Can only be observed when a substance is changed into another substance. • Flammability, corrosiveness, reactivity with acid, etc. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 19. Types of Properties • Intensive Properties… – Are independent of the amount of the substance that is present. • Density, boiling point, color, etc. • Extensive Properties… – Depend upon the amount of the substance present. • Mass, volume, energy, etc. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 20. Types of Changes • Physical Changes – These are changes in matter that do not change the composition of a substance. • Changes of state, temperature, volume, etc. • Chemical Changes – Chemical changes result in new substances. • Combustion, oxidation, decomposition, etc. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 21. Chemical Reactions In the course of a chemical reaction, the reacting substances are converted to new Matter substances. And Measurement © 2009, Prentice-Hall, Inc.
- 22. Compounds Compounds can be broken down into more elemental particles. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 23. Separation of Mixtures Matter And Measurement © 2009, Prentice-Hall, Inc.
- 24. Filtration In filtration solid substances are separated from liquids and solutions. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 25. Distillation Distillation uses differences in the boiling points of substances to separate a homogeneous mixture into its components. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 26. Chromatography This technique separates substances on the basis of differences in solubility in a solvent. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 27. What do these countries have in common? US, Liberia and Burma Matter And Measurement © 2009, Prentice-Hall, Inc.
- 28. What do these countries have in common? US, Liberia and Burma • They use the imperial system Matter And Measurement © 2009, Prentice-Hall, Inc.
- 29. View of Countries using Metric USA Berma Liberia Matter And Measurement © 2009, Prentice-Hall, Inc.
- 30. Units of Measurement Matter And Measurement © 2009, Prentice-Hall, Inc.
- 31. SI Units • Système International d’Unités • A different base unit is used for each quantity. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 32. Metric System Prefixes convert the base units into units that are appropriate for the item being measured. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 33. Volume • The most commonly used metric units for volume are the liter (L) and the milliliter (mL). – A liter is a cube 1 dm long on each side. – A milliliter is a cube 1 cm long on each side. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 34. Uncertainty in Measurement Matter And Measurement © 2009, Prentice-Hall, Inc.
- 35. Uncertainty in Measurements Different measuring devices have different uses and different degrees of accuracy. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 36. Uncertainty in Measurements Different measuring devices have different uses and different degrees of accuracy. 1 ml Matter And Measurement © 2009, Prentice-Hall, Inc.
- 37. Uncertainty in Measurements Different measuring devices have different uses and different degrees of accuracy. 0.1 ml Matter And Measurement © 2009, Prentice-Hall, Inc.
- 38. Accuracy versus Precision • Accuracy refers to the proximity of a measurement to the true value of a quantity. • Precision refers to the proximity of several measurements to each other. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 39. Significant Figures • The term significant figures refers to digits that were measured. • When rounding calculated numbers, we pay attention to significant figures so we do not overstate the accuracy of our answers. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 40. Significant Figures 1. All nonzero digits are significant. 2. Zeroes between two significant figures are themselves significant. 3. Zeroes at the beginning of a number are never significant. 4. Zeroes at the end of a number are significant if a decimal point is written in the number. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 41. Significant Figures • When addition or subtraction is performed, answers are rounded to the least significant decimal place. • When multiplication or division is performed, answers are rounded to the number of digits that corresponds to the least number of significant figures in any of the numbers used in the calculation. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 42. Temperature By definition temperature is a measure of the average kinetic energy of the particles in a sample. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 43. Temperature • In scientific measurements, the Celsius and Kelvin scales are most often used. • The Celsius scale is based on the properties of water. – 0°C is the freezing point of water. – 100°C is the boiling point of water. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 44. Temperature • The Kelvin is the SI unit of temperature. • It is based on the properties of gases. • There are no negative Kelvin temperatures. • K = °C + 273.15 Matter And Measurement © 2009, Prentice-Hall, Inc.
- 45. Temperature • The Fahrenheit scale is not used in scientific measurements. ∀ °F = 9/5(°C) + 32 ∀ °C = 5/9(°F − 32) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 46. Density Density is a physical property of a substance. m d= V Matter And Measurement © 2009, Prentice-Hall, Inc.
- 47. Dimensional Analysis • We use dimensional analysis to convert one quantity to another. • Most commonly dimensional analysis utilizes conversion factors (e.g., 1 in. = 2.54 cm) 1 in. 2.54 cm or 2.54 cm 1 in. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 48. Dimensional Analysis Use the form of the conversion factor that puts the sought-for unit in the numerator. desired unit Given unit × = desired unit given unit Conversion factor Matter And Measurement © 2009, Prentice-Hall, Inc.
- 49. Dimensional Analysis • For example, to convert 8.00 m to inches, – convert m to cm – convert cm to in. 100 cm 1 in. 8.00 m × × = 315 in. 1m 2.54 cm Matter And Measurement © 2009, Prentice-Hall, Inc.
- 50. Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter reemerged in the early 19th century, championed by John Dalton. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 51. Dalton's Postulates Each element is composed of extremely small particles called atoms. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 52. Dalton's Postulates All atoms of a given element are identical to one another in mass (?) and other properties, but the atoms of one element are different from the atoms of all other elements. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 53. Dalton's Postulates Atoms of an element are not changed into atoms of a different element by chemical reactions; atoms are neither created nor destroyed in chemical reactions. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 54. Dalton’s Postulates Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 55. Law of Constant Composition Joseph Proust (1754–1826) • This is also known as the law of definite proportions. • It states that the elemental composition of a pure substance never varies. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 56. Law of Conservation of Mass The total mass of substances present at the end of a chemical process is the same as the mass of substances present before the process took place. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 57. The Electron • Streams of negatively charged particles were found to emanate from cathode tubes. • J. J. Thompson is credited with their discovery (1897). Matter And Measurement © 2009, Prentice-Hall, Inc.
- 58. The Electron Thompson measured the charge/mass ratio of the electron to be 1.76 × 108 coulombs/g. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 59. Millikan Oil Drop Experiment Once the charge/mass ratio of the electron was known, determination of either the charge or the mass of an electron would yield the other. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 60. Millikan Oil Drop Experiment Robert Millikan (University of Chicago) determined the charge on the electron in 1909. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 61. Radioactivity • Radioactivity is the spontaneous emission of radiation by an atom. • It was first observed by Henri Becquerel. • Marie and Pierre Curie also studied it. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 62. Radioactivity • Three types of radiation were discovered by Ernest Rutherford: α particles β particles γ rays Matter And Measurement © 2009, Prentice-Hall, Inc.
- 63. The Atom, circa 1900 • The prevailing theory was that of the “plum pudding” model, put forward by Thompson. • It featured a positive sphere of matter with negative electrons imbedded in it. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 64. Discovery of the Nucleus Ernest Rutherford shot α particles at a thin sheet of gold foil and observed the pattern of scatter of the particles. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 65. The Nuclear Atom Since some particles were deflected at large angles, Thompson’s model could not be correct. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 66. The Nuclear Atom • Rutherford postulated a very small, dense nucleus with the electrons around the outside of the atom. • Most of the volume of the atom is empty space. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 67. Other Subatomic Particles • Protons were discovered by Rutherford in 1919. • Neutrons were discovered by James Chadwick in 1932. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 68. Subatomic Particles • Protons and electrons are the only particles that have a charge. • Protons and neutrons have essentially the same mass. • The mass of an electron is so small we ignore it. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 69. Symbols of Elements Elements are symbolized by one or two letters. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 70. Atomic Number All atoms of the same element have the same number of protons: The atomic number (Z) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 71. Atomic Mass The mass of an atom in atomic mass units (amu) is the total number of protons and neutrons in the atom. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 72. Isotopes • Isotopes are atoms of the same element with different masses. • Isotopes have different numbers of neutrons. 11 12 13 14 6 C 6 C 6 C 6 C Matter And Measurement © 2009, Prentice-Hall, Inc.
- 73. Atomic Mass Atomic and molecular masses can be measured with great accuracy with a mass spectrometer. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 74. Average Mass • Because in the real world we use large amounts of atoms and molecules, we use average masses in calculations. • Average mass is calculated from the isotopes of an element weighted by their relative abundances. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 75. Periodic Table • It is a systematic catalog of the elements. • Elements are arranged in order of atomic number. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 76. Periodicity When one looks at the chemical properties of elements, one notices a repeating pattern of reactivities. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 77. Periodic Table • The rows on the periodic chart are periods. • Columns are groups. • Elements in the same group have similar chemical properties. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 78. Groups These five groups are known by their names. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 79. Periodic Table Nonmetals are on the right side of the periodic table (with the exception of H). Matter And Measurement © 2009, Prentice-Hall, Inc.
- 80. Periodic Table Metalloids border the stair-step line (with the exception of Al, Po, and At). Matter And Measurement © 2009, Prentice-Hall, Inc.
- 81. Periodic Table Metals are on the left side of the chart. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 82. Chemical Formulas The subscript to the right of the symbol of an element tells the number of atoms of that element in one molecule of the compound. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 83. Chemical Formulas Molecular compounds are composed of molecules and almost always contain only nonmetals. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 84. Diatomic Molecules These seven elements occur naturally as molecules containing two atoms. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 85. Types of Formulas • Empirical formulas give the lowest whole-number ratio of atoms of each element in a compound. • Molecular formulas give the exact number of atoms of each element in a compound. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 86. Types of Formulas • Structural formulas show the order in which atoms are bonded. • Perspective drawings also show the three-dimensional array of atoms in a compound. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 87. Ions • When atoms lose or gain electrons, they become ions. – Cations are positive and are formed by elements on the left side of the periodic chart. – Anions are negative and are formed by elements Matter on the right side of the periodic chart. And Measurement © 2009, Prentice-Hall, Inc.
- 88. Ionic Bonds Ionic compounds (such as NaCl) are generally formed between metals and nonmetals. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 89. Writing Formulas • Because compounds are electrically neutral, one can determine the formula of a compound this way: – The charge on the cation becomes the subscript on the anion. – The charge on the anion becomes the subscript on the cation. – If these subscripts are not in the lowest whole- number ratio, divide them by the greatest common Matter factor. And Measurement © 2009, Prentice-Hall, Inc.
- 90. Common Cations Matter And Measurement © 2009, Prentice-Hall, Inc.
- 91. Common Anions Matter And Measurement © 2009, Prentice-Hall, Inc.
- 92. Inorganic Nomenclature • Write the name of the cation. • If the anion is an element, change its ending to -ide; if the anion is a polyatomic ion, simply write the name of the polyatomic ion. • If the cation can have more than one possible charge, write the charge as a Roman numeral in parentheses. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 93. Patterns in Oxyanion Nomenclature • When there are two oxyanions involving the same element: – The one with fewer oxygens ends in -ite. • NO2− : nitrite; SO32− : sulfite – The one with more oxygens ends in -ate. • NO3− : nitrate; SO42− : sulfate Matter And Measurement © 2009, Prentice-Hall, Inc.
- 94. Patterns in Oxyanion Nomenclature • The one with the second fewest oxygens ends in -ite. – ClO2− : chlorite • The one with the second most oxygens ends in -ate. – ClO3− : chlorate Matter And Measurement © 2009, Prentice-Hall, Inc.
- 95. Patterns in Oxyanion Nomenclature • The one with the fewest oxygens has the prefix hypo- and ends in -ite. – ClO− : hypochlorite • The one with the most oxygens has the prefix per- and ends in -ate. – ClO4− : perchlorate Matter And Measurement © 2009, Prentice-Hall, Inc.
- 96. Acid Nomenclature • If the anion in the acid ends in -ide, change the ending to -ic acid and add the prefix hydro- . – HCl: hydrochloric acid – HBr: hydrobromic acid – HI: hydroiodic acid Matter And Measurement © 2009, Prentice-Hall, Inc.
- 97. Acid Nomenclature • If the anion in the acid ends in -ite, change the ending to -ous acid. – HClO: hypochlorous acid – HClO2: chlorous acid Matter And Measurement © 2009, Prentice-Hall, Inc.
- 98. Acid Nomenclature • If the anion in the acid ends in -ate, change the ending to -ic acid. – HClO3: chloric acid – HClO4: perchloric acid Matter And Measurement © 2009, Prentice-Hall, Inc.
- 99. Nomenclature of Binary Compounds • The less electronegative atom is usually listed first. • A prefix is used to denote the number of atoms of each element in the compound (mono- is not used on the first element listed, however) . Matter And Measurement © 2009, Prentice-Hall, Inc.
- 100. Nomenclature of Binary Compounds • The ending on the more electronegative element is changed to -ide. – CO2: carbon dioxide – CCl4: carbon tetrachloride Matter And Measurement © 2009, Prentice-Hall, Inc.
- 101. Nomenclature of Binary Compounds • If the prefix ends with a or o and the name of the element begins with a vowel, the two successive vowels are often elided into one. N2O5: dinitrogen pentoxide Matter And Measurement © 2009, Prentice-Hall, Inc.
- 102. Nomenclature of Organic Compounds • Organic chemistry is the study of carbon. • Organic chemistry has its own system of nomenclature. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 103. Nomenclature of Organic Compounds The simplest hydrocarbons (compounds containing only carbon and hydrogen) are alkanes. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 104. Nomenclature of Organic Compounds The first part of the names above correspond to the number of carbons (meth- = 1, eth- = 2, prop- = 3, etc.). Matter And Measurement © 2009, Prentice-Hall, Inc.
- 105. Nomenclature of Organic Compounds • When a hydrogen in an alkane is replaced with something else (a functional group, like -OH in the compounds above), the name is derived from the name of the alkane. • The ending denotes the type of compound. Matter – An alcohol ends in -ol. And Measurement © 2009, Prentice-Hall, Inc.
- 106. Law of Conservation of Mass “We may lay it down as an incontestable axiom that, in all the operations of art and nature, nothing is created; an equal amount of matter exists both before and after the experiment. Upon this principle, the whole art of performing chemical experiments depends.” --Antoine Lavoisier, 1789 Matter And Measurement © 2009, Prentice-Hall, Inc.
- 107. Chemical Equations Chemical equations are concise representations of chemical reactions. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 108. Anatomy of a Chemical Equation CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 109. Anatomy of a Chemical Equation CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g) Reactants appear on the left side of the equation. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 110. Anatomy of a Chemical Equation CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g) Products appear on the right side of the equation. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 111. Anatomy of a Chemical Equation CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g) The states of the reactants and products are written in parentheses to the right of Matter And each compound. Measurement © 2009, Prentice-Hall, Inc.
- 112. Anatomy of a Chemical Equation CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g) Coefficients are inserted to balance the equation. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 113. Subscripts and Coefficients Give Different Information • Subscripts tell the number of atoms of each element in a molecule. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 114. Subscripts and Coefficients Give Different Information • Subscripts tell the number of atoms of each element in a molecule • Coefficients tell the number of Matter And molecules. Measurement © 2009, Prentice-Hall, Inc.
- 115. Reaction Types Matter And Measurement © 2009, Prentice-Hall, Inc.
- 116. Combination Reactions • In this type of reaction two or more substances react to form one product. • Examples: – 2 Mg (s) + O2 (g) → 2 MgO (s) – N2 (g) + 3 H2 (g) → 2 NH3 (g) Matter – C3H6 (g) + Br2 (l) → C3H6Br2 (l) And Measurement © 2009, Prentice-Hall, Inc.
- 117. Decomposition Reactions • In a decomposition one substance breaks down into two or more substances. • Examples: – CaCO3 (s) → CaO (s) + CO2 (g) – 2 KClO3 (s) → 2 KCl (s) + O2 (g) Matter – 2 NaN3 (s) → 2 Na (s) + 3 N2 (g) And Measurement © 2009, Prentice-Hall, Inc.
- 118. Combustion Reactions • These are generally rapid reactions that produce a flame. • Most often involve hydrocarbons reacting with oxygen in the air. • Examples: – CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (g) Matter – C3H8 (g) + 5 O2 (g) → 3 CO2 (g) + 4 H2O (g) And Measurement © 2009, Prentice-Hall, Inc.
- 119. Formula Weights Matter And Measurement © 2009, Prentice-Hall, Inc.
- 120. Formula Weight (FW) • A formula weight is the sum of the atomic weights for the atoms in a chemical formula. • So, the formula weight of calcium chloride, CaCl2, would be Ca: 1(40.1 amu) + Cl: 2(35.5 amu) 111.1 amu • Formula weights are generally reported for ionic compounds. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 121. Molecular Weight (MW) • A molecular weight is the sum of the atomic weights of the atoms in a molecule. • For the molecule ethane, C2H6, the molecular weight would be C: 2(12.0 amu) + H: 6(1.0 amu) 30.0 amu Matter And Measurement © 2009, Prentice-Hall, Inc.
- 122. Percent Composition One can find the percentage of the mass of a compound that comes from each of the elements in the compound by using this equation: (number of atoms)(atomic weight) % element = x 100 (FW of the compound) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 123. Percent Composition So the percentage of carbon in ethane is… (2)(12.0 amu) %C = (30.0 amu) 24.0 amu = x 100 30.0 amu = 80.0% Matter And Measurement © 2009, Prentice-Hall, Inc.
- 124. Moles Matter And Measurement © 2009, Prentice-Hall, Inc.
- 125. Avogadro’s Number • 6.02 x 1023 • 1 mole of 12C has a mass of 12 g. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 126. Molar Mass • By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Matter And Measurement © 2009, Prentice-Hall, Inc.
- 127. Using Moles Moles provide a bridge from the molecular scale to the real-world scale. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 128. Mole Relationships • One mole of atoms, ions, or molecules contains Avogadro’s number of those particles. • One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 129. Finding Empirical Formulas Matter And Measurement © 2009, Prentice-Hall, Inc.
- 130. Calculating Empirical Formulas One can calculate the empirical formula from the percent composition. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 131. Calculating Empirical Formulas The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61.31%), hydrogen (5.14%), nitrogen (10.21%), and oxygen (23.33%). Find the empirical formula of PABA. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 132. Calculating Empirical Formulas Assuming 100.00 g of para-aminobenzoic acid, C: 61.31 g x 1 mol = 5.105 mol C 12.01 g 1 mol H: 5.14 g x = 5.09 mol H 1.01 g 1 mol N: 10.21 g x = 0.7288 mol N 14.01 g 1 mol O: 23.33 g x = 1.456 mol O 16.00 g Matter And Measurement © 2009, Prentice-Hall, Inc.
- 133. Calculating Empirical Formulas Calculate the mole ratio by dividing by the smallest number of moles: 5.105 mol C: = 7.005 ≈ 7 0.7288 mol 5.09 mol H: = 6.984 ≈ 7 0.7288 mol 0.7288 mol N: = 1.000 0.7288 mol 1.458 mol O: = 2.001 ≈ 2 Matter 0.7288 mol And Measurement © 2009, Prentice-Hall, Inc.
- 134. Calculating Empirical Formulas These are the subscripts for the empirical formula: C7H7NO2 Matter And Measurement © 2009, Prentice-Hall, Inc.
- 135. Combustion Analysis • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this. – C is determined from the mass of CO2 produced. – H is determined from the mass of H2O produced. – O is determined by difference after the C and H have been determined. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 136. Elemental Analyses Compounds containing other elements are analyzed using methods analogous to those used for C, H and O. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 137. Stoichiometric Calculations The coefficients in the balanced equation give the ratio of moles of reactants and products. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 138. Stoichiometric Calculations Starting with the mass of Substance A you can use the ratio of the coefficients of A and B to calculate the mass of Substance B formed (if it’s a product) or used (if it’s a reactant). Matter And Measurement © 2009, Prentice-Hall, Inc.
- 139. Stoichiometric Calculations C6H12O6 + 6 O2 → 6 CO2 + 6 H2O Starting with 1.00 g of C6H12O6… we calculate the moles of C6H12O6… use the coefficients to find the moles of H2O… Matter and then turn the moles of water to grams. And Measurement © 2009, Prentice-Hall, Inc.
- 140. Limiting Reactants Matter And Measurement © 2009, Prentice-Hall, Inc.
- 141. How Many Cookies Can I Make? • You can make cookies until you run out of one of the ingredients. • Once this family runs out of sugar, they will stop making cookies (at least any cookies you would want to eat). Matter And Measurement © 2009, Prentice-Hall, Inc.
- 142. How Many Cookies Can I Make? • In this example the sugar would be the limiting reactant, because it will limit the amount of cookies you can make. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 143. Limiting Reactants • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). Matter And Measurement © 2009, Prentice-Hall, Inc.
- 144. Limiting Reactants In the example below, the O2 would be the excess reagent. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 145. Theoretical Yield • The theoretical yield is the maximum amount of product that can be made. – In other words it’s the amount of product possible as calculated through the stoichiometry problem. • This is different from the actual yield, which is the amount one actually produces and measures. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 146. Percent Yield One finds the percent yield by comparing the amount actually obtained (actual yield) to the amount it was possible to make (theoretical yield). Actual Yield Percent Yield = x 100 Theoretical Yield Matter And Measurement © 2009, Prentice-Hall, Inc.
- 147. Solutions • Solutions are defined as homogeneous mixtures of two or more pure substances. • The solvent is present in greatest abundance. • All other substances are solutes. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 148. Dissociation • When an ionic substance dissolves in water, the solvent pulls the individual ions from the crystal and solvates them. • This process is called dissociation. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 149. Dissociation • An electrolyte is a substances that dissociates into ions when dissolved in water. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 150. Electrolytes • An electrolyte is a substances that dissociates into ions when dissolved in water. • A nonelectrolyte may dissolve in water, but it does not dissociate into ions when it does Matter so. And Measurement © 2009, Prentice-Hall, Inc.
- 151. Electrolytes and Nonelectrolytes Soluble ionic compounds tend to be electrolytes. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 152. Electrolytes and Nonelectrolytes Molecular compounds tend to be nonelectrolytes, except for acids and bases. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 153. Electrolytes • A strong electrolyte dissociates completely when dissolved in water. • A weak electrolyte only dissociates partially when dissolved in water. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 154. Strong Electrolytes Are… • Strong acids • Strong bases Matter And Measurement © 2009, Prentice-Hall, Inc.
- 155. Strong Electrolytes Are… • Strong acids • Strong bases • Soluble ionic salts Matter And Measurement © 2009, Prentice-Hall, Inc.
- 156. Precipitation Reactions When one mixes ions that form compounds that are insoluble (as could be predicted by the solubility guidelines), a precipitate is formed. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 157. Metathesis (Exchange) Reactions • Metathesis comes from a Greek word that means “to transpose.” AgNO3 (aq) + KCl (aq) → AgCl (s) + KNO3 (aq) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 158. Metathesis (Exchange) Reactions • Metathesis comes from a Greek word that means “to transpose.” • It appears the ions in the reactant compounds exchange, or transpose, ions. AgNO3 (aq) + KCl (aq) → AgCl (s) + KNO3 (aq) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 159. Solubility of different compounds (NS = non soluble in water, S = soluble in water) Cl- Br- I- NO3- SO42- CO32- PO43- Li+ S S S S S S S S Na+ S S S S S S S S K+ S S S S S S S S Mg2+ NS S S S S S NS NS Ca2+ S S S S S S NS NS Sr2+ S S S S S NS NS NS Ba2+ S S S S S NS NS NS Fe2+ NS S S S S S NS NS Fe3+ NS S S S S S NS NS Ni2+ NS S S S S S NS NS Cu+ NS S S S S S NS NS Cu2+ NS S S S S S NS NS Al3+ NS S S S S S NS NS Zn2+ NS S S S S S NS NS Ag+ NS NS NS NS S S NS NS Pb2+ NS NS NS NS S NS NS NS Matter And Measurement
- 160. Solution Chemistry • It is helpful to pay attention to exactly what species are present in a reaction mixture (i.e., solid, liquid, gas, aqueous solution). • If we are to understand reactivity, we must be aware of just what is changing during the course of a reaction. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 161. Molecular Equation The molecular equation lists the reactants and products in their molecular form. AgNO3 (aq) + KCl (aq) → AgCl (s) + KNO3 (aq) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 162. Ionic Equation • In the ionic equation all strong electrolytes (strong acids, strong bases, and soluble ionic salts) are dissociated into their ions. • This more accurately reflects the species that are found in the reaction mixture. Ag+ (aq) + NO3- (aq) + K+ (aq) + Cl- (aq) → AgCl (s) + K+ (aq) + NO3- (aq) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 163. Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) → Matter AgCl (s) + K+(aq) + NO3-(aq) And Measurement © 2009, Prentice-Hall, Inc.
- 164. Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. • The only things left in the equation are those things that change (i.e., react) during the course of the reaction. Ag+(aq) + Cl-(aq) → AgCl (s) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 165. Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. • The only things left in the equation are those things that change (i.e., react) during the course of the reaction. • Those things that didn’t change (and were deleted from the net ionic equation) are called spectator ions. Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) → Matter AgCl (s) + K+(aq) + NO3-(aq) And Measurement © 2009, Prentice-Hall, Inc.
- 166. Writing Net Ionic Equations 1. Write a balanced molecular equation. 2. Dissociate all strong electrolytes. 3. Cross out anything that remains unchanged from the left side to the right side of the equation. 4. Write the net ionic equation with the species that remain. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 167. Acids • Arrhenius defined acids as substances that increase the concentration of H+ when dissolved in water. • Brønsted and Lowry defined them as proton donors. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 168. Acids There are only seven strong acids: • Hydrochloric (HCl) • Hydrobromic (HBr) • Hydroiodic (HI) • Nitric (HNO3) • Sulfuric (H2SO4) • Chloric (HClO3) • Perchloric (HClO4) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 169. Bases • Arrhenius defined bases as substances that increase the concentration of OH− when dissolved in water. • Brønsted and Lowry defined them as proton acceptors. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 170. Bases The strong bases are the soluble metal salts of hydroxide ion: • Alkali metals • Calcium • Strontium • Barium Matter And Measurement © 2009, Prentice-Hall, Inc.
- 171. Acid-Base Reactions In an acid-base reaction, the acid donates a proton (H+) to the base. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 172. Neutralization Reactions Generally, when solutions of an acid and a base are combined, the products are a salt and water. CH3COOH (aq) + NaOH (aq) →CH3COONa (aq) + H2O (l) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 173. Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 174. Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) → Na+ (aq) + Cl- (aq) + H2O (l) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 175. Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) → Na+ (aq) + Cl- (aq) + H2O (l) H+ (aq) + OH- (aq) → H2O (l) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 176. Gas-Forming Reactions • Some metathesis reactions do not give the product expected. • In this reaction, the expected product (H2CO3) decomposes to give a gaseous product (CO2). CaCO3 (s) + HCl (aq) →CaCl2 (aq) + CO2 (g) + H2O (l) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 177. Gas-Forming Reactions When a carbonate or bicarbonate reacts with an acid, the products are a salt, carbon dioxide, and water. CaCO3 (s) + HCl (aq) →CaCl2 (aq) + CO2 (g) + H2O (l) NaHCO3 (aq) + HBr (aq) →NaBr (aq) + CO2 (g) + H2O (l) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 178. Gas-Forming Reactions Similarly, when a sulfite reacts with an acid, the products are a salt, sulfur dioxide, and water. SrSO3 (s) + 2 HI (aq) →SrI2 (aq) + SO2 (g) + H2O (l) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 179. Gas-Forming Reactions • This reaction gives the predicted product, but you had better carry it out in the hood, or you will be very unpopular! • But just as in the previous examples, a gas is formed as a product of this reaction. Na2S (aq) + H2SO4 (aq) → Na2SO4 (aq) + H2S (g) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 180. Oxidation-Reduction Reactions • An oxidation occurs when an atom or ion loses electrons. • A reduction occurs when an atom or ion gains electrons. • One cannot occur without the other. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 181. Oxidation Numbers To determine if an oxidation-reduction reaction has occurred, we assign an oxidation number to each element in a neutral compound or charged entity. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 182. Oxidation Numbers • Elements in their elemental form have an oxidation number of 0. • The oxidation number of a monatomic ion is the same as its charge. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 183. Oxidation Numbers • Nonmetals tend to have negative oxidation numbers, although some are positive in certain compounds or ions. Oxygen has an oxidation number of −2, except in the peroxide ion in which it has an oxidation number of −1. Hydrogen is −1 when bonded to a metal, +1 when bonded to a nonmetal. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 184. Oxidation Numbers • Nonmetals tend to have negative oxidation numbers, although some are positive in certain compounds or ions. Fluorine always has an oxidation number of −1. The other halogens have an oxidation number of −1 when they are negative; they can have positive oxidation numbers, however, most notably in oxyanions. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 185. Oxidation Numbers • The sum of the oxidation numbers in a neutral compound is 0. • The sum of the oxidation numbers in a polyatomic ion is the charge on the ion. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 186. Displacement Reactions • In displacement reactions, ions oxidize an element. • The ions, then, are reduced. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 187. Displacement Reactions In this reaction, silver ions oxidize copper metal. Cu (s) + 2 Ag+ (aq) → Cu2+ (aq) + 2 Ag (s) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 188. Displacement Reactions The reverse reaction, however, does not occur. x Cu2+ (aq) + 2 Ag (s) → Cu (s) + 2 Ag+ (aq) Matter And Measurement © 2009, Prentice-Hall, Inc.
- 189. Activity Series Matter And Measurement © 2009, Prentice-Hall, Inc.
- 190. Molarity • Two solutions can contain the same compounds but be quite different because the proportions of those compounds are different. • Molarity is one way to measure the concentration of a solution. moles of solute Molarity (M) = volume of solution in liters Matter And Measurement © 2009, Prentice-Hall, Inc.
- 191. Mixing a Solution • To create a solution of a known molarity, one weighs out a known mass (and, therefore, number of moles) of the solute. • The solute is added to a volumetric flask, and solvent is added to the line on the neck of the flask. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 192. Dilution • One can also dilute a more concentrated solution by – Using a pipet to deliver a volume of the solution to a new volumetric flask, and – Adding solvent to the line on the neck of the new flask. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 193. Dilution The molarity of the new solution can be determined from the equation Mc × Vc = Md × Vd, where Mc and Md are the molarity of the concentrated and dilute solutions, respectively, and Vc and Vd are the volumes of the two solutions. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 194. Using Molarities in Stoichiometric Calculations Matter And Measurement © 2009, Prentice-Hall, Inc.
- 195. Titration Titration is an analytical technique in which one can calculate the concentration of a solute in a solution. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 196. Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 Thermochemistry John D. Bookstaver Matter St. Charles Community College And Cottleville, MO Measurement © 2009, Prentice-Hall, Inc.
- 197. Energy • Energy is the ability to do work or transfer heat. – Energy used to cause an object that has mass to move is called work. – Energy used to cause the temperature of an object to rise is called heat. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 198. Potential Energy Potential energy is energy an object possesses by virtue of its position or chemical composition. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 199. Kinetic Energy Kinetic energy is energy an object possesses by virtue of its motion. 1 KE = mv2 2 Matter And Measurement © 2009, Prentice-Hall, Inc.
- 200. Units of Energy • The SI unit of energy is the joule (J). kg m2 1 J = 1 s2 • An older, non-SI unit is still in widespread use: the calorie (cal). 1 cal = 4.184 J Matter And Measurement © 2009, Prentice-Hall, Inc.
- 201. Definitions: System and Surroundings • The system includes the molecules we want to study (here, the hydrogen and oxygen molecules). • The surroundings are everything else (here, the cylinder and piston). Matter And Measurement © 2009, Prentice-Hall, Inc.
- 202. Definitions: Work • Energy used to move an object over some distance is work. • w=F×d where w is work, F is the force, and d is the distance over which the force is exerted. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 203. Heat • Energy can also be transferred as heat. • Heat flows from warmer objects to cooler objects. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 204. Conversion of Energy • Energy can be converted from one type to another. • For example, the cyclist above has potential energy as she sits on top of the hill. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 205. Conversion of Energy • As she coasts down the hill, her potential energy is converted to kinetic energy. • At the bottom, all the potential energy she had at the top of the hill is now kinetic energy. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 206. First Law of Thermodynamics • Energy is neither created nor destroyed. • In other words, the total energy of the universe is a constant; if the system loses energy, it must be gained by the surroundings, and vice versa. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 207. Internal Energy The internal energy of a system is the sum of all kinetic and potential energies of all components of the system; we call it E. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 208. Internal Energy By definition, the change in internal energy, ∆E, is the final energy of the system minus the initial energy of the system: ∆E = Efinal − Einitial Matter And Measurement © 2009, Prentice-Hall, Inc.
- 209. Changes in Internal Energy • If ∆E > 0, Efinal > Einitial – Therefore, the system absorbed energy from the surroundings. – This energy change is called endergonic. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 210. Changes in Internal Energy • If ∆E < 0, Efinal < Einitial – Therefore, the system released energy to the surroundings. – This energy change is called exergonic. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 211. Changes in Internal Energy • When energy is exchanged between the system and the surroundings, it is exchanged as either heat (q) or work (w). • That is, ∆E = q + w. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 212. ∆E, q, w, and Their Signs Matter And Measurement © 2009, Prentice-Hall, Inc.
- 213. Exchange of Heat between System and Surroundings • When heat is absorbed by the system from the surroundings, the process is endothermic. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 214. Exchange of Heat between System and Surroundings • When heat is absorbed by the system from the surroundings, the process is endothermic. • When heat is released by the system into the surroundings, the process is exothermic. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 215. State Functions Usually we have no way of knowing the internal energy of a system; finding that value is simply too complex a problem. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 216. State Functions • However, we do know that the internal energy of a system is independent of the path by which the system achieved that state. – In the system below, the water could have reached room temperature from either direction. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 217. State Functions • Therefore, internal energy is a state function. • It depends only on the present state of the system, not on the path by which the system arrived at that state. • And so, ∆E depends only on Einitial and Efinal. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 218. State Functions • However, q and w are not state functions. • Whether the battery is shorted out or is discharged by running the fan, its ∆E is the same. – But q and w are different in the two cases. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 219. Work Usually in an open container the only work done is by a gas pushing on the surroundings (or by the surroundings pushing on the gas). Matter And Measurement © 2009, Prentice-Hall, Inc.
- 220. Work We can measure the work done by the gas if the reaction is done in a vessel that has been fitted with a piston. w = -P∆V Matter And Measurement © 2009, Prentice-Hall, Inc.
- 221. Enthalpy • If a process takes place at constant pressure (as the majority of processes we study do) and the only work done is this pressure-volume work, we can account for heat flow during the process by measuring the enthalpy of the system. • Enthalpy is the internal energy plus the product of pressure and volume: H = E + PV Matter And Measurement © 2009, Prentice-Hall, Inc.
- 222. Enthalpy • When the system changes at constant pressure, the change in enthalpy, ∆H, is ∆H = ∆(E + PV) • This can be written ∆H = ∆E + P∆V Matter And Measurement © 2009, Prentice-Hall, Inc.
- 223. Enthalpy • Since ∆E = q + w and w = -P∆V, we can substitute these into the enthalpy expression: ∆H = ∆E + P∆V ∆H = (q+w) − w ∆H = q • So, at constant pressure, the change in enthalpy is the heat gained or lost. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 224. Endothermicity and Exothermicity • A process is endothermic when ∆H is positive. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 225. Endothermicity and Exothermicity • A process is endothermic when ∆H is positive. • A process is exothermic when ∆H is negative. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 226. Enthalpy of Reaction The change in enthalpy, ∆H, is the enthalpy of the products minus the enthalpy of the reactants: ∆H = Hproducts − Hreactants Matter And Measurement © 2009, Prentice-Hall, Inc.
- 227. Enthalpy of Reaction This quantity, ∆H, is called the enthalpy of reaction, or the heat of reaction. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 228. The Truth about Enthalpy • Enthalpy is an extensive property. • ∆H for a reaction in the forward direction is equal in size, but opposite in sign, to ∆H for the reverse reaction. • ∆H for a reaction depends on the state of the products and the state of the reactants. Matter And Measurement © 2009, Prentice-Hall, Inc.
- 229. Calorimetry Since we cannot know the exact enthalpy of the reactants and products, we measure ∆H through calorimetry, the measurement of heat flow. Matter And Measurement © 2009, Prentice-Hall, Inc.
Notes de l'éditeur
- Figure 2.1 John Dalton (1766-1844)
- Figure 2.1 John Dalton (1766-1844)
- Figure 2.1 John Dalton (1766-1844)
- Figure 2.1 John Dalton (1766-1844)
- Figure 2.4
- Figure 2.4
- Figure 2.5
- Figure 2.5
- Figure 2.8
- Figure 2.9
- Figure 2.10
- Figure 2.11
- Figure 2.12
- Table 2.1
- Figure 2.13