1 redox (1)
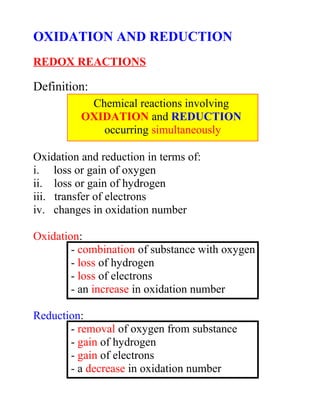
- 1. OXIDATION AND REDUCTION REDOX REACTIONS Definition: Oxidation and reduction in terms of: i. loss or gain of oxygen ii. loss or gain of hydrogen iii. transfer of electrons iv. changes in oxidation number Oxidation: - combination of substance with oxygen - loss of hydrogen - loss of electrons - an increase in oxidation number Reduction: - removal of oxygen from substance - gain of hydrogen - gain of electrons - a decrease in oxidation number Chemical reactions involving OXIDATION and REDUCTION occurring simultaneously
- 2. Oxidant / Oxidizing agent: The substance that causes oxidation Reductant / Reducing agent: The substance that causes reduction Important Neutralization and Precipitation are NOT redox reactions WHY? You tell me [wait until we discuss the ‘oxidation number’]
- 3. A. Loss or gains of oxygen Oxidation: - combination of substance with oxygen Reduction: - removal of oxygen from substance Example: 2CuO + C 2Cu + CO2 CuO loses its oxygen to form copper. Carbon gains the oxygen to form carbon dioxide. CuO causes the oxidation of carbon. Carbon causes the reduction of CuO. Material undergoes oxidation: Carbon, C Material undergoes reduction: Copper(II) oxide, CuO Oxidizing agent / oxidant : Copper(II) oxide, CuO Reducing agent / reductant : Carbon, C
- 4. B. Loss or gains of hydrogen Oxidation: - loss of hydrogen Reduction: - gain of hydrogen Example: H2S + Cl2 S + 2HCl H2S loses its hydrogen to form sulfur. Cl2 gains the hydrogen to form HCl. H2S causes the reduction of Cl2. Cl2 causes the oxidation of H2S. Material undergoes oxidation : Hydrogen sulphide, H2S Material undergoes reduction : Chlorine, Cl2 Oxidizing agent / oxidant : Chlorine, Cl2 Reducing agent / reductant : Hydrogen sulphide, H2S
- 5. C. Tranfer of electrons Oxidation: - loss of electrons Reduction: - gain of electrons Example 1: [Daniell cell] Zn + Cu2+ Zn2+ + Cu Electrons transfer from zinc to copper(II) ions. Half reaction: One zinc atom loses 2 electrons to form one zinc ion. Zinc is oxidized to zinc ions. Oxidizing half-equation: Zn Zn2+ + 2e- Half reaction: One copper(II) ion gains 2 electron to form one copper atom. Copper(II) ion is reduced to copper. Reduction half-equation: Cu2+ + 2e Cu
- 6. Copper(II) ions act as oxidizing agent because it accepts electrons. Zinc acts as reducing agent because it releases electrons. Oxidizing half-equation: Zn Zn2+ + 2e- Reduction half-equation: Cu2+ + 2e- Cu [balanced the number of electron please] Zn + Cu2+ + 2e- Cu + Zn2+ + 2e- Thus; Ionic Equation : Zn + Cu2+ Zn2+ + Cu Chemical equation: Zn + CuSO4 ZnSO4 + Cu [note: the sum of the two half-equations gives the ionic equation] Cancel the electrons
- 7. D. Changes in oxidation number Oxidation number? Definition: The oxidation number of an element is the charge that the atom of the element would have if complete transfer of electron occurs Rules: pg 107 figure 3.1 (read) Tips from the rules: • the oxidation number for atom and molecule is zero • the oxidation number for monoatomic ion is equal to its charge. • the sum of oxidation numbers of all elements in the compound is zero • the sum of oxidation number of all elements in polyatomic ions is equal to the charge of the ions Oxidation: - an increase in oxidation number Reduction:
- 8. - a decrease in oxidation number Example: [ I use the same example but from different perspective] Zn + Cu2+ Zn2+ + Cu [easy way to detect which substance is oxidized or reduced] The oxidation number of zinc, Zn increases from 0 to +2. Zn undergoes oxidation to zinc ions, Zn2+ . The oxidation number of copper(II) ions, Cu2+ decreases from +2 to 0. Cu2+ undergoes reduction to copper, Cu. Copper(II) ions, Cu2+ act as oxidizing agent. Zinc, Zn acts as reducing agent. Oxidizing half-equation: Zn Zn2+ + 2e- Reduction half-equation: Cu2+ + 2e- Cu Ionic Equation : Zn + Cu2+ Zn2+ + Cu [note: every redox reaction MUST have half equations +2 0 Oxidation number Zn Zn2+ Cu Cu2+ Oxidation (loses electrons) Reduction (gains electrons)
- 9. and ionic equation] Example: 2Mg + O2 2MgO Describe the process…….. The oxidation number of magnesium, Mg increases from 0 to +2. Mg undergoes oxidation to magnesium ions, Mg2+ . Oxidizing half-equation: Mg Mg2+ + 2e- 1 magnesium atom loses 2 electrons to from 1 mzgnesium ions. Mg undergoes oxidation to magnesium ions, Mg2+ . The oxidation number of oxygen, O2 decreases from 0 to -2. O2 undergoes reduction to oxide ions, O2- . +2 0 Oxidation number Mg Mg2+ O2- O2 Oxidation (loses electrons) Reduction (gains electrons) -2
- 10. Reduction half-equation: O2 + 4e- 2O2- 1 molecule of oxygen gains 4 electron to form 2 oxide ions. O2 undergoes reduction to oxide ions, O2- . Oxygen, O2 act as oxidizing agent. Magnesium, Mg acts as reducing agent. Oxidizing half-equation: Mg Mg2+ + 2e- (×2) [it becomes: 2Mg 2Mg2+ + 4e] Reduction half-equation: O2 + 4e- 2O2- [balanced the number of electrons for both half equation] 2Mg + O2 + 4e- 2Mg2+ + 2O2- + 4e- Thus; Ionic Equation: 2Mg + O2 2Mg2+ + 2O2- or 2Mg + O2 2MgO HW: pg 110 Learning task 3.1 Analyzing Cancel the electrons
- 11. [a and b] Explain each of the reaction Solution: (a) (i) 2H2 + O2 2H2O [rule: pg 107] The oxidation number of hydrogen, H2 increases from 0 to +1. H2 undergoes oxidation to hydrogen ion, H+ . Oxidizing half-equation: H2 2H+ + 2e- 1 molecule of hydrogen loses 2 electrons to form 2 hydrogen ions. The oxidation number of oxygen, O2 decreases from 0 to -2. O2 undergoes reduction to oxide ions, O2- . Reduction half-equation: O2 + 4e- 2O2- 1 molecule of oxygen gains 4 electrons to form +1 0 Oxidation number H2 H+ O2- O2 Oxidation (loses electrons) Reduction (gains electrons) -2
- 12. 2 oxide ions. Oxygen, O2 act as oxidizing agent. Hydrogen, H2 acts as reducing agent. Oxidizing half-equation: H2 2H+ + 2e- (×2) Reduction half-equation: O2 + 4e- 2O2- [balanced the number of electrons for both half equation] 2H2 + O2 + 4e- 4H+ + 2O2- + 4e- Thus; The ionic equation 2H2 + O2 4H+ + 2O2- or 2H2 + O2 2H2O Easy lah! Prepared by; Kamal Ariffin Bin Saaim SMKDBL Cancel the electrons
