Chain Reactions
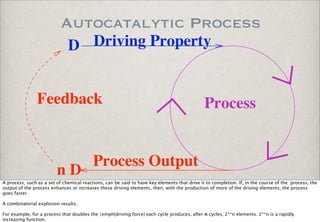
- 1. Autocatalytic Process D Driving Property Feedback Process Process Output nD A process, such as a set of chemical reactions, can be said to have key elements that drive it to completion. If, in the course of the process, the output of the process enhances or increases these driving elements, then, with the production of more of the driving elements, the process goes faster. A combinatorial explosion results. For example, for a process that doubles the emph{driving force} each cycle produces, after n cycles, 2**n elements. 2**n is a rapidly increasing function.
- 2. Ignition Process The driving forces are: •Heat (Temperature) •The breaking of bonds produces heat • Heat increases temperature • Higher temperature makes the reactions faster •Radicals •More radicals make the system more reactive •The more radicals •The faster the reactions Within an ignition process, there are basically two driving forces, acting simultaneously, which make the process autocatalytic: - Heat (or Temperature) - Radicals Heat release, caused for example by the breaking of bonds, can create a temperature increase, which in turn can drive the reactions to react faster, starting the catalytic cycle again. Radicals are very reactive. The more radicals a system have, the more reactive the system is in general. This, in turn, produces more radicals, promoting the catalytic cycle. Heat release and radicals both increase the overall rate of the process. In a typical hydrocarbon combustion process, both are usually increasing at the same time, especially when considering a zero-dimensional system, where only the chemical reactions are considered. In high dimensions, di!usion or conductivity can also promote the increase of radicals or heat, respectively.
- 3. Thermal Explosions A simple system: •Heat Source: •A set of chemical reactions •Heat Sink: •Surrounding wall at constant temperature If the heat generated by the reactions (increasing T) is faster than the walls can absorb Then heat is accumulated in the system Explosion occurs In the next couple of slides a simple system will be analyzed where heat is generated by a set of reactions and some of the heat is absorbed by the walls. If the heat generated is faster than the absorption by the walls, then the system accumulates heat and an explosion is possible.
- 4. Closed System 3"&')76):"&;'<745 :"&;'<745 :)*) ! -)= !"#$"%&'(%")*)! 3"&')!%&456"%)'7)8&99 +)*),)-)./012)-).!!!&2) !"#$"%&'(%")*)!& To examine the problem a bit more quantitatively, a simple system is set up: A set of exothermic reactions occur within a closed vessel with the walls at a certain temperature. The chemical reactions, with rates dependent on temperature, produce heat and contribute to increase the temperature of the system. The walls of the system, are kept at a certain temperature. How much heat they absorb or give o! is proportional to the di!erence in temperature within the vessel and the walls.
- 5. Equations: Wall • Heat Transfer to Wall: L = h(S/V)(T-Ta) • The heat transfered, L, • at a temperature, T, • to the wall at temperature, Ta, • which is a function of the surface area, S, and • volume, V • is scaled by the heat transfer coefficient, h, The amount of heat that is conducted between one system and another, in this case the wall and inside of the vessel, is proportional to the di!erence between the system temperature and the wall temperature. One can also imagine that the amount of heat is also proportional to the amount of contact between the wall and the inside of the vessel where the reactions occur. Hence the term, S/V, per unit volume. In addition, the amount of heat transfer is dependent on the substances involved. Thus there is a heat transfer coe"cient. The sign of the temperature determines the direction of the heat transfer.
- 6. Equations: Reactions • Heat Production by Reactions: R = ! q • The heat produced, R, • with a reaction rate of !(1/(conc time)) and • a net molar exothermicity, q. The heat, R, produced by the reactions, can be represented simply by the net rate of reaction and the net molar heat production.
- 7. Effect of Heat Transfer •Heat Transfer to Wall: L = h(S/V)(T-Ta) •Heat Production by Reactions: R = ! q •R > L: Temperature increase •Walls don’t absorb enough heat •R< L: Temperature decrease •Walls absorb more heat than produced Two cases can be isolated. If the heat release of the reactions is greater than the absorption of the walls, the wall won’t absorb enough heat and the temperature increases. If the wall absorption is greater, then heat is taken out of the system and there is a temperature decrease.
- 8. Heat Sources and Sinks Increasing Concentration Heat Release by Reacion Heat Transfer to Walls Tu Tc Ta Ts Temperature The heat release of a set of reactions is dependent on temperature. This is depicted as the red line in the diagram. As the temperature increases, the amount of heat released increases. Note that it is not linear. The walls, acting as a large heat sink, try to stabilize the temperature to the given temperature. With no source of heat, the temperature within the vessel would tend toward the wall temperature. The blue line represents the rate of heat that the wall is capable of absorbing from the system. Below the blue line, the rate of heat absorption of the wall is greater, so the system tends toward the temperature of the wall, because the wall can absorb heat faster.
- 9. Temperature Increase Temperature Walls absorb less heat than reactions produce Heat Release by Reacion Heat Transfer to Walls Heat put into system So a increase in T Until absorbsion and production are equal 1 2 3 4 Reactants Walls Heat Absorb Heat In the case that the reactions produce more heat than the walls can absorb, then it can be expected that the temperature increases. However, in this case, the temperature increases until, once again, the reactions produce the same amount of heat as the walls absorb. In both cases, the system stabilizes so the wall and the system have the same temperature.
- 10. Wall Sink Temperature Walls absorb more heat than reactions produce Heat Release by Reacion Heat Transfer to Walls Heat taken out So a decrease in T Until absorbsion and production are equal 6 5 4 3 2 1 Reactants Walls Heat Absorb Heat With the case that the wall absorbs more heat than the reactions produce, then it can be expected that thermal explosion does not occur. The walls keep the process in check. If the walls absorb more heat, then the overall temperature of the process decreases. Progressively, from step one to step 6, the temperature settles to where the heat loss to the walls equals the heat produced by the reactions.
- 11. Thermal Explosion Heat Release by Reacion Heat Transfer to Walls R >L Ta Temperature Alway Temperature Increase In the case that the heat produced by the reactions is always larger than the heat absorbed by the walls then the temperature will always increase and a thermal explosion occurs.
- 12. Isothermal Chain Branching Initiation: A −→ X ν1 = k1 [A] Branching: A + X −→ mX + products ν2 = k2 [X][A] Termination: X −→ products ν3 = k3 [X] The last example was explosion due to increase in temperature (how the reactions produced the rise in temperature was not specified. In this example, the temperature is kept constant and the driving force is a molecular species. The initiation reactions get the chain reactions going, usually by creating the driving force species. For example, an initiation reaction can be one with no radicals as reactants, but create radicals as products. With radicals being the driving force. The branching reactions are those which produce more of the driving force species. For example, if the reactant contain one radical, but the products contain two (not necessarily the same) radical, then the reaction is a branching reaction. This type of reaction enhances the catalytic action. If the same number of radicals are found in the products and reactions, then it could be called a propagation reaction. Termination steps reduce the driving force. For example, if two radicals combine to form a non-radical species, then it is a terminal reaction.
- 13. Chain Equations d[X] = k1 [A] + (m − 1)k2 [A][X] − k3 [X] dt Pool Approximation [A] = [A]0 d[X] = k1 [A]0 + (m − 1)k2 [A]0 [X] − k3 [X] dt Writing the full expression for the production of X, if we assume that the amount of fuel, A, is always in abundance, then we can assume that concentration is always close to the initial concentration. This is called the pool approximation and is at least true at the beginning of the explosion. The final expression becomes an expression whose only dependent variable is [X].
- 14. Chain Equations d[X] = k1 [A]0 + [X]((m − 1)k2 [A]0 − k3 ) = k1 [A]0 + φ[X] dt Integrating: k1 [A]0 φt [X] = (e − 1) φ with φ = (m − 1)k2 [A]0 − k3 With this approximation, d[X]/dt is only a function of X, so the expression can be integrated to give an expression for [X]: [X] = k1[A0]/!(exp(!t) - 1) The critical parameter is ! in the exponential. If we examine the values, we can divide the behavior into di"erent regimes.
- 15. Case: ! < 0 Case 1: φ < 0 k3 > (m − 1)k2 [A]0 Termination stronger k1 [A]0 [X] → φ Reaches stable value Looking at the value of !, the behavior of the system can be analyzed through the behavior of [X] as t approaches infinity. If ! is less than 0, the termination reaction, k3, is larger than the branching. This means that the system will reach a stable value.
- 16. Case: ! > 0 Case 2: φ > 0 k3 < (m − 1)k2 [A]0 Branching stronger [X] → inf grows without limit, explodes In the case of ! > 0, the termination reactions are slower, the branching reactions dominate, so the value of [X] grows without limit. The system explodes.
- 17. Case: ! = 0 Case 3: φ = 0 k3 = (m − 1)k2 [A]0 Branching and Termination balanced [X] = k1 [A] linear growth In the case of ! = 0, the branching and termination reactions are balanced and [X] has linear growth of [X] = k1[A].
- 18. Isothermal Chain Branching !!%!# !!$!# [X] !!"!# time This is a graph of the behavior of [X] versus time of the the three cases represented by ! = (m-1)k_2[A0]-k3, where ! gives the relationship between branching reaction, in the term (m-1)k2[A0] and the termination reaction, in the term k3. So in this case, at constant temperature conditions, it is purely the species X which determines the explosion. In a typical combustion reaction, actually it is a combination of both which produces the autocatalytic e"ect. In fact, they enhance can each other. As the temperature goes up, the rates of reactions increase, increasing the number of radicals in the system.
- 19. Ignition Process: Time Scale Very fast Equilibrium Temperature Ignition Initiation time Unburnt Burn (Equilibrium) State State Approximation for high temperature processes The autocatalytic e!ect in a typical combustion ignition is very fast. The rise in temperature can be very steep. In higher temperature conditions, there is a slow rise in temperature for the initiation period and then when a certain threshold is reached, then there is a very rapid rise in temperature. Also in terms of time scales, one can almost view the process as a step function, where the species are either in the initial unburnt configuration or, assuming ignition occurred, in the burnt configuration.
