Properties of Ammonia
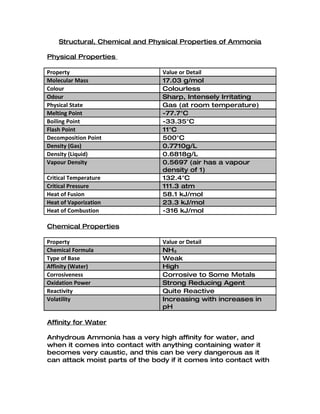
- 1. Structural, Chemical and Physical Properties of Ammonia Physical Properties Property Value or Detail Molecular Mass 17.03 g/mol Colour Colourless Odour Sharp, Intensely Irritating Physical State Gas (at room temperature) Melting Point -77.7°C Boiling Point -33.35°C Flash Point 11°C Decomposition Point 500°C Density (Gas) 0.7710g/L Density (Liquid) 0.6818g/L Vapour Density 0.5697 (air has a vapour density of 1) Critical Temperature 132.4°C Critical Pressure 111.3 atm Heat of Fusion 58.1 kJ/mol Heat of Vaporization 23.3 kJ/mol Heat of Combustion -316 kJ/mol Chemical Properties Property Value or Detail Chemical Formula NH3 Type of Base Weak Affinity (Water) High Corrosiveness Corrosive to Some Metals Oxidation Power Strong Reducing Agent Reactivity Quite Reactive Volatility Increasing with increases in pH Affinity for Water Anhydrous Ammonia has a very high affinity for water, and when it comes into contact with anything containing water it becomes very caustic, and this can be very dangerous as it can attack moist parts of the body if it comes into contact with
- 2. them. Despite the high affinity for water, ammonia has limited reactivity with water. Chemical Reactivity The combustion of ammonia proceeds with difficulty but yields nitrogen gas and water. 4NH + 3O + heat 2N + 6H O However, with the use of a catalyst and under the correct conditions of temperature--as described above in Oxyacids of nitrogen and their salts--ammonia reacts with oxygen to produce nitric oxide, NO, which is oxidized to nitrogen dioxide, NO , and is used in the industrial synthesis of nitric acid. Ammonia readily dissolves in water with the liberation of heat. NH + H O NH + + OH- These aqueous solutions of ammonia are basic and are sometimes called solutions of ammonium hydroxide (NH OH). The equilibrium, however, is such that a 1.0 molar solution of NH provides only 4.2 millimoles of hydroxide ion. The hydrates NH H O, 2NH H O, and NH 2H O exist and have been shown to consist of ammonia and water molecules linked by intermolecular hydrogen bonds. Liquid ammonia is used extensively as a nonaqueous solvent. The alkali metals as well as the heavier alkaline earth metals and even some inner transition metals dissolve in liquid ammonia, producing blue solutions. Physical measurements, including electrical conductivity studies, provide evidence that this blue colour and electrical current are due to the solvated electron. These solutions are excellent sources of electrons for reducing other chemical species. As the concentration of dissolved metal increases, the solution becomes a deeper blue in colour and finally changes to a copper-coloured solution with a metallic lustre. The electrical conductivity decreases, and there is evidence that the solvated electrons associate to form electron pairs. Most ammonium salts also readily dissolve in liquid ammonia.
- 3. Ammonia in Redox Ammonia gas is a strong reducing agent When dry ammonia gas is passed over heated black copper oxide it will be reduced to brown copper. 3CUO + 2NH3 3Cu +N2 + 3H2O It reduces lead monoxide to lead 3PbO 2NH3 3Pb + N2 + 3H2O Ammonia reduces chlorine to hydrogen chloride 8NH3 + 3Cl2 6NH4Cl + N2 If chlorine is in excess NH3 + 3Cl2 3HCl + NCl3 (nitrogen tri-chloride) When a glass rod dipped in concentrated hydrochloric acid is brought near ammonia it gives out dense white fumes of ammonium chloride which is the confirmative test for ammonia NH3 + HCl NH4Cl Reaction with Nessler’s reagent K2 HgI4 When mercuric chloride is added to potassium iodide solution scarlet red precipitate form which dissolves in excess of mercuric chloride and become a clear solution without any colour. This is known as Nesslers reagent. When ammonia is passed over the Nesslers reagent it turns pale brown. On passing excess of ammonia over it a brown precipitate is obtained. Structural Properties
- 4. Ammonia consists of one Nitrogen atom bonded covalently to three Hydrogen atoms, as can be seen in the above diagram. The shape of the molecule can be said to be a triangular pyramid and is dipolar.
