Sandrogreco Tabela De P Ka
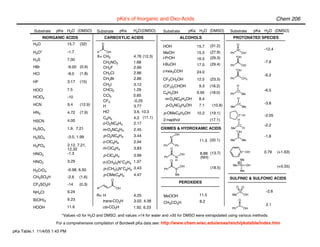
- 1. pKa's of Inorganic and Oxo-Acids Chem 206 Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) INORGANIC ACIDS CARBOXYLIC ACIDS ALCOHOLS PROTONATED SPECIES H2O 15.7 (32) O (31.2) O HOH 15.7 N+ -12.4 H3O+ -1.7 X OH MeOH 15.5 (27.9) Ph OH X= CH3 4.76 (12.3) i-PrOH (29.3) + OH H2S 7.00 16.5 CH2NO2 1.68 -7.8 t-BuOH 17.0 (29.4) Ph OH HBr -9.00 (0.9) CH2F 2.66 + c-hex3COH OH HCl -8.0 (1.8) CH2Cl 2.86 24.0 -6.2 CH2Br 2.86 CF3CH2OH 12.5 (23.5) Ph CH3 HF 3.17 (15) CH2I 3.12 (CF3)2CHOH 9.3 (18.2) H HOCl 7.5 CHCl2 1.29 O+ -6.5 C6H5OH 9.95 (18.0) Ph Me HClO4 -10 CCl3 0.65 CF3 -0.25 m-O2NC6H4OH 8.4 H HCN 9.4 (12.9) O+ -3.8 H 3.77 p-O2NC6H4OH 7.1 (10.8) Me Me HN3 4.72 (7.9) HO 3.6, 10.3 p-OMeC6H4OH 10.2 (19.1) O+ H -2.05 C6H5 4.2 (11.1) HSCN 4.00 2-napthol (17.1) H o-O2NC6H4 2.17 O+ -2.2 H2SO3 1.9, 7.21 m-O2NC6H4 2.45 OXIMES & HYDROXAMIC ACIDS Me + H OH p-O2NC6H4 3.44 OH H2SO4 -3.0, 1.99 N S -1.8 o-ClC6H4 2.94 11.3 (20.1) Me Me H3PO4 2.12, 7.21, Ph Ph 12.32 m-ClC6H4 3.83 O N+ OH 0.79 (+1.63) HNO3 -1.3 OH 8.88 (13.7) p-ClC6H4 3.99 Ph N (NH) O H Me HNO2 3.29 o-(CH3)3N+C6H4 1.37 OH Me N OH (+5.55) p-(CH3)3N+C6H4 3.43 Ph N (18.5) H2CrO4 -0.98, 6.50 Me p-OMeC6H4 4.47 Me CH3SO3H -2.6 (1.6) SULFINIC & SULFONIC ACIDS O PEROXIDES CF3SO3H -14 (0.3) R OH O O NH4Cl 9.24 S -2.6 R= H 4.25 MeOOH 11.5 Me OH B(OH)3 9.23 trans-CO2H 3.02, 4.38 O CH3CO3H 8.2 S 2.1 HOOH 11.6 cis-CO2H 1.92, 6.23 Ph OH *Values <0 for H2O and DMSO, and values >14 for water and >35 for DMSO were extrapolated using various methods. For a comprehensive compilation of Bordwell pKa data see: http://www.chem.wisc.edu/areas/reich/pkatable/index.htm pKa Table.1 11/4/05 1:43 PM
- 2. D.H. Ripin, D.A. Evans pKa's of Nitrogen Acids Chem 206 Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) PROTONATED NITROGEN AMINES IMIDES HYDROXAMIC ACID & AMIDINES HN3 4.7 (7.9) O O O N+H4 9.2 (10.5) 8.88 (13.7) NH3 38 (41) OH (NH) EtN+H 3 10.6 NH 8.30 NH (14.7) Ph N i-Pr2NH (36 THF)) H i-Pr2N+H2 11.05 TMS2NH 26(THF) (30) NSO2Ph O O R= Me (17.3) Et3N+H 10.75 (9.00) PhNH2 (30.6) Ac2NH (17.9) Ph (15.0) R NH2 PhN+H3 4.6 (3.6) Ph2NH (25.0) Me Me SULFONAMIDE PhN+(Me) 5.20 (2.50) NCNH2 (16.9) HETEROCYCLES 2H NH (37) RSO2NH2 R = Me (17.5) Ph2N+H2 0.78 NH (44) Ph (16.1) H (20.95) H Me CF3 6.3 (9.7) N N (16.4) 2-napthal-N+H3 4.16 Me MeSO2NHPh (12.9) H2NN+H3 8.12 H2N N (26.5) H N GUANIDINIUM, N (11.9) HON+H3 5.96 HYRDAZONES,- IDES, & -INES N NH (23.0) H AMIDES & CARBAMATES N+H2 N Quinuclidine N+ 11.0 (9.80) (13.6) NNH2 (21.6) O R= H (23.5) Me2N NMe2 Ph Me X= O (24) N CH3 15.1 (25.5) HN Morpholine O N+H2 8.36 O (18.9) N X X= S (13.3) (18.6) R NH2 Ph (23.3) H Ph NHNH2 X N-Me morpholine 7.38 CF3 (17.2) NO2 PhSO2NHNH2 (17.2) X= O (14.8) (urea) NH2 (26.9) NH PhNHNHPh X= S (11.8) N + OEt (24.8) (26.1) N (13.9) O2N NH3 -9.3 N O O PROTONATED HETEROCYCLES H X Ph 12 (20.5) X= O (24.4) NO2 Et N N H (21.6) O NH N X= S (27.0) H (12) (estimate) N N N+ H DBU (19.8) 2.97, 8.82 + H DABCO N+ (2.97, 8.93) O Bn N O H S S H n= 1 (24.1) (20.8) DMAP H (29.4) H (16.5) H3N+ NH + +NH 6.90, 9.95 n= 2 (26.4) O NH + NH N N+ 3 ( )n Me2N NH 9.2 6.95 Me Me +NH +NH HN 3 3 i-Pr -9.0, 12.0 O R Me O 5.21 (3.4) R= H (PPTS) NMe N Proton Sponge (--, 7.50) (15) (24) O 4.95 (0.90)+ t-Bu H H O NH NH N (12.1) N + (18.4) N+ H 6.75 (4.46) Me Me Me PhCN+H -10 R 0.72 Cl, H i-Pr *Values <0 for H2O and DMSO, and values >14 for water and >35 for DMSO were extrapolated using various methods. For a comprehensive compilation of Bordwell pKa data see: http://www.chem.wisc.edu/areas/reich/pkatable/index.htm pKa Table.2 11/4/05 1:43 PM
- 3. D.H. Ripin, D.A. Evans pKa's of CH bonds in Hydrocarbons and Carbonyl Compounds Chem 206 Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) O HYDROCARBONS ESTERS KETONES (Me)3CH O O Me 53 24.5 (30.3) X (Me)2CH2 51 t-BuO Me Me X O (26.5) X= H CH2=CH2 Ph (23.6) Ph (19.8) X= H (24.7) 50 t-BuO (18.7) (25.7) O SPh OMe CH4 48 (56) NMe2 (27.5) (20.0) COCH3 9 (13.3) N+Me3 (23.8) 46 EtO SO2Ph (12.5) Br O O O CN (22.0) CH2=CHCH3 43 (44) 11 (14.2) 19-20 (27.1) O EtO Me Et Et O PhH 43 O O 13 (15.7) (28.3) n PhCH3 (43) i-Pr O i-Pr 41 MeO OMe O (27.7) Ph2CH2 33.5 (32.2) t-Bu O Me n= 4 (25.1) S (20.9) (25.8) MeO (26.3) 5 Ph3CH 31.5 (30.6) S Ph i-Pr 6 (26.4) HCCH 24 O O 7 (27.7) Ph [30.2 (THF)] 8 (27.4) PhCCH 23 (28.8) X LiO Ph XC6H4CH3 X= H (24.7) AMIDES CH3 (24.4) (28.1) X= p-CN (30.8) O O (26.6) Ph (17.7) p-NO2 (20.4) Ph COCH3 (14.2) Me2N O COPh (13.3) (29.0) p-COPh (26.9) (25.9) O SPh CN (10.2) Me Me Me2N O F (21.6) (26.1) (24.9) (22.85) O N+Me3 OMe (25.5) Me Me Et2N O OPh (21.1) SPh (16.9) CN 20 (20.1) N (17.2) SePh (18.6) NPh2 (20.3) O O O (32.4) 15 (18.0) (18.2) N+Me3 (14.6) Me2N Me NO2 (7.7) Me Me S H2 ~36 (25.7) SO2Ph (11.4) Me2N Me *Values <0 for H2O and DMSO, and values >14 for water and >35 for DMSO were extrapolated using various methods. For a comprehensive compilation of Bordwell pKa data see: http://www.chem.wisc.edu/areas/reich/pkatable/index.htm pKa Table.3 11/4/05 1:44 PM
- 4. D.H. Ripin, D.A. Evans pKa's of CH bonds at Nitrile, Heteroaromatic, and Sulfur Substituted Carbon Chem 206 Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) NITRILES SULFIDES SULFOXIDES SULFONES O O O NC X PhSCH2X S X S X X= Ph (30.8) Me Ph X= H (31.3) CN (20.8) X= H (35.1) (29.0) Ph (29.0) X= H CH3 (32.5) COCH3 (18.7) SPh (29.0) CH3 (31.0) (21.9) COPh (16.9) O t-Bu (31.2) Ph (10.2) NO2 (11.8) S X Ph (23.4) COPh (30.8) SPh Ph CH=CH2 (22.5) CONR2 (17.1) SO2Ph (20.5) X= H (33) CH=CHPh (20.2) CO2Et (13.1) Ph (27.2) (22.1) SO2CF3 (11.0) (18.2) CCH POPh2 (24.9) SOPh (17.8) CN 11 (11.1) O CCPh COPh (11.4) OPh (28.1) MeSCH2SO2Ph (23.4) S (24.5) Ph CHPh2 COMe (12.5) N+Me3 (20.6) PhSCHPh2 (26.7) OPh (27.9) SPh (20.8) (PhS)3CH (22.8) SULFONIUM N+Me3 (19.4) CN (12.0) SO2Ph (12.0) (PrS)3CH (31.3) Me3S+=O (18.2) NO2 (7.1) Me SMe (23.5) Me (16.3) (20.5) HETERO-AROMATICS S SPh S+ (30.5) Ph CH2Ph SO2Ph (12.2) S S PPh2 (20.2) (28.2) H Ph SULFIMIDES & SULFOXIMINES O O N (PhS)2CHPh (23.0) S (22.3) NTs Ph CHPh2 S (30.1) X S O O N Ph Ph R (31.1) S S R= Me (27.6) Me Me N X= Ph (30.7) i-Pr (30.7) O O (26.7) O NTs (18.8) Ph CO2Me (20.8) S S (24.5) CF3 Me CN (19.1) Ph Me O O RSCH2CN O NMe S (21.8) Ph (24.3) (33) CF3 i-Pr N+ (25.2) R= Me S O O Ph Me Et (24.0) N+Me2 S (26.6) O- O (23.6) (14.4) CF3 i-Pr S Ph (30.2) (22.9) Ph Me O O O t-Bu O NTs (32.8) S PhSCH=CHCH2SPh (26.3) S (20.7) Et Et Ph (30.0) Ph CH2Cl S BuSH 10-11 (17.0) (PhSO2)2CH2Me (14.3) PhSH ≈7 (10.3) *Values <0 for H2O and DMSO, and values >14 for water and >35 for DMSO were extrapolated using various methods. pKa Table.4 11/4/05 1:44 PM
- 5. D. H. Ripin, D. A. Evans pKa's of CH bonds at Heteroatom Substituted Carbon & References Chem 206 Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) Substrate pKa H2O (DMSO) REFERENCES ETHERS PHOSPHONIUM NITRO DMSO: P+H4 -14 RNO2 CH3OPh (49) JACS 97, 7007 (1975) MeP+H3 2.7 R= CH3 ≈10 (17.2) JACS 97, 7160 (1975) MeOCH2SO2Ph (30.7) Et3P+H 9.1 CH2Me (16.7) JACS 97, 442 (1975) PhOCH2SO2Ph (27.9) JACS 105, 6188 (1983) Ph3P+CH3 (22.4) CHMe2 (16.9) JOC 41, 1883 (1976) PhOCH2CN (28.1) Ph3P+i-Pr (21.2) CH2Ph (12.2) JOC 41, 1885 (1976) O JOC 41, 2786 (1976) Ph3P+CH2COPh (6.2) CH2Bn (16.2) MeO (22.85) JOC 41, 2508 (1976) Ph Ph3P+CH2CN (7.0) CH2SPh (11.8) JOC 42, 1817 (1977) CH2SO2Ph (7.1) JOC 42, 321 (1977) CH2COPh (7.7) JOC 42, 326 (1977) SELENIDES PHOSPONATES & JOC 43, 3113 (1978) PHOSPHINE OXIDES O2N JOC 43, 3095 (1978) O O JOC 43, 1764 (1978) n JOC 45, 3325 (1980) PhSe (18.6) (EtO)2P X Ph JOC 45, 3305 (1980) X= Ph (27.6) (26.9) JOC 45, 3884 (1980) PhSeCHPh2 (27.5) n= 3 JOC 46, 4327 (1981) CN (16.4) (PhSe)2CH2 (31.3) 4 (17.8) JOC 46, 632 (1981) CO2Et (18.6) JOC 47, 3224 (1982) 5 (16.0) PhSeCH2Ph (31.0) Cl (26.2) JOC 47, 2504 (1982) 6 (17.9) Acc. Chem. Res. 21, 456 (1988) PhSeCH=CHCH2SePh (27.2) SiMe3 (28.8) (15.8) Unpublished results of F. Bordwell O 7 Ph2P X Water: AMMONIUM IMINES X= SPh (24.9) Advanced Org. Chem., 3rd Ed. N Ph J. March (1985) Me3N+CH2X CN (16.9) (24.3) Unpublished results of W. P. Jencks X= CN (20.6) Ph Ph SO2Ph (19.4) PHOSPHINES Oxime ethers are ~ 10 pka units less THF: (14.6) acidic than their ketone counterparts JACS 110, 5705 (1988) COPh Streitwieser, JOC 1991, 56, 1989 CO2Et (20.0) Ph2PCH2PPh2 (29.9) See cited website below for additional data CONEt2 (24.9) Ph2PCH2SO2Ph (20.2) *Values <0 for H2O and DMSO, and values >14 for water and >35 for DMSO were extrapolated using various methods. For a comprehensive compilation of Brodwell pKa data see: http://www.chem.wisc.edu/areas/reich/pkatable/index.htm pKa Table.5 11/4/05 1:45 PM
- 6. DMSO Acidities of Common Heterocycles Bordwell, ACR, 1988, 21, 456 Bordwell http://www.chem.wisc.edu/areas/reich/pkatable/index.htm N N N N N N N N N N N N N H H H H H H 23.0 19.8 18.6 16.4 13.9 11.9 18.0 O O O O O O O N N N N N O N O H H H H H H 24.0 20.8 15.0 12.1 26.4 24.0 Me O S Me Me S Me S N N H H H H N+ N S N Me N+ N+ H N N Me Me H H Me Me Me 13.3 14.8 11.8 29.4 16.5 18.4 24 Pka Table.6.cdx 11/4/05 1:45 PM
