Here are the steps to make up the requested molar solutions:1. Dissolve 58.44 g NaCl in 1 L water2. Dissolve 116.88 g NaCl in 500 mL water 3. Dissolve 98 g H2SO4 in 1 L water4. Dissolve 19.6 g H2SO4 in 200 mL water5. Dissolve 100 g KOH in 2.5 L water6. Dissolve 36.46 g MgCl2 in 300 mL water7. Dissolve 342 g sucrose in 500 mL water8. Dissolve 180 g glucose in 400 mL water
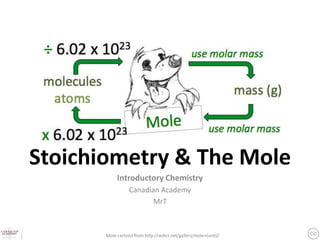
- 1. Stoichiometry & The Mole Introductory Chemistry Canadian Academy MrT Mole cartoon from http://wdict.net/gallery/mole+(unit)/
- 2. 2
- 3. How many candies in a mole of candies? Image: 'Color Overload' http://www.flickr.com/photos/40645538@N00/4911180896
- 4. How many candies in a mole of candies? 6.02 x 10 23 Avogadro’s Number Image: 'Color Overload' http://www.flickr.com/photos/40645538@N00/4911180896
- 5. How many grains of sand in a mole of grains of sand? Image: 'Desert Textures' http://www.flickr.com/photos/43355249@N00/2243862024
- 6. How many grains of sand in a mole of grains of sand? 6.02 x 10 23 Avogadro’s Number Image: 'Desert Textures' http://www.flickr.com/photos/43355249@N00/2243862024
- 7. How many chickens in a mole of chickens? Image: ’Chickens raised for meat (by PETA)' http://www.veganoutreach.org/whyvegan/animals.html
- 8. How many chickens in a mole of chickens? 6.02 x 10 23 Avogadro’s Number Image: ’Chickens raised for meat (by PETA)' http://www.veganoutreach.org/whyvegan/animals.html
- 9. How many Beliebers in a mole of Beliebers? Image: 'Justin Bieber's fans' http://www.flickr.com/photos/33166549@N05/6219319308
- 10. How many Beliebers in a mole of Beliebers? 6.02 x 10 23 Avogadro’s Number Image: 'Justin Bieber's fans' http://www.flickr.com/photos/33166549@N05/6219319308
- 11. How many moles in a mole of moles? Image: 'Day 94 - Mole' http://www.flickr.com/photos/85088843@N00/446305760
- 12. How many moles in a mole of moles? 6.02 x 10 23 Avogadro’s Number Image: 'Day 94 - Mole' http://www.flickr.com/photos/85088843@N00/446305760
- 13. The Mole 6.02 x 1023 A word used to describe a number. Just like a dozen describes 12 objects or a score describes 20 objects. Avogadro’s Number This standard description helps us take massive numbers (or tiny things) and make them manageable. One mole of atoms of an element is 1 x 6 x 1023 atoms One mole of molecules of a compound is 1 x 6 x 1023 molecules
- 14. The Mole 6.02 x 1023 A word used to describe a number. Just like a dozen describes 12 objects or a score describes 20 objects. Avogadro’s Number This standard description helps us take massive numbers (or tiny things) and make them manageable. 6020000000000 0000000000000 that’s a lot of zeroes
- 15. What do chemists use to make guaca-mole? 15 Guacamole image, from http://en.wikipedia.org/wiki/Guacamole
- 16. What do chemists use to make guaca-mole? Avogadros Chemistry Cat @professorkitteh 16 Guacamole image, from http://en.wikipedia.org/wiki/Guacamole
- 17. Using the Mole 6.02 x 10 23 Avogadro’s Number One mole of molecules of water H2 O
- 18. Using the Mole 6.02 x 10 23 Avogadro’s Number One mole of molecules of water Contains… H2 O Two moles of atoms One mole of atoms of hydrogen of oxygen
- 19. Using the Mole 6.02 x 10 23 Avogadro’s Number 6.02 x 1023 molecules of water Contains… H2 O 2 x 6.02 x 10 23 1 x 6.02 x 1023 atoms of hydrogen atoms of oxygen
- 20. Using the Mole 6.02 x 10 23 unit = mol-1 e.g. g mol-1 = grams per mole (g/mol) Avogadro’s Number 6.02 x 1023 molecules of water Contains… H2 O 2 x 6.02 x 10 23 1 x 6.02 x 1023 atoms of hydrogen atoms of oxygen
- 21. Electrolysis of water gas 1 gas 2 faster bubbling slower bubbling Cathode Anode Explain two ways in which your could deduce which gas was in each test tube AND: one way to test the gases experimentally.
- 22. Electrolysis of water 2H2O (l) electricity 2H2 + O2 (g) (g) There’s a lot going on in that plastic cup! http://www.sepuplhs.org/high/hydrogen/electrolysis_sim.html
- 23. What do you call a tooth in a glass of water? A one-molar solution! But it’s not really, is it? • The tooth has not dissolved, so it is not a solution. Chemistry Cat Tooth from: http://photo-dictionary.com/phrase/7639/molar-tooth.html#b @professorkitteh 23 Glass of water from: http://openclipart.org/detail/132019/glass-of-water-by-gustavorezende
- 24. Molar Solutions are solutions with a known concentration of solute molecules. A one-molar solution Is one mole of the solute (element or compound) dissolved into one litre (1dm3) of water. So how would you make up a 1M solution of HCl? 24 Glass of water from: http://openclipart.org/detail/132019/glass-of-water-by-gustavorezende
- 25. Molar Solutions are solutions with a known concentration of solute molecules. A one-molar solution Is one mole of the solute (element or compound) dissolved into one litre (1dm3) of water. So how would you make up a 1M solution of HCl? 1. Calculate the molar mass of HCl. • 1.01g + 35.45g = 36.46g 2. Measure out this mass on a balance. 3. Dissolve in 1l distilled water. 25 Glass of water from: http://openclipart.org/detail/132019/glass-of-water-by-gustavorezende
- 26. A one-molar solution Is one mole of the solute (element or compound) dissolved into one litre (1dm3) of water. So how would you make up these solutions? 1. 1L 1M NaCl 2. 500mL 2M NaCl 3. 1L 1M H2SO4 4. 200ml 0.2M H2SO4 5. 2.5L 0.4M KOH 6. 300mL 1M MgCl2 7. 500mL 2M sucrose solution 8. 400mL 3M glucose solution 26 Glass of water from: http://openclipart.org/detail/132019/glass-of-water-by-gustavorezende
- 27. A one-molar solution Is one mole of the solute (element or compound) dissolved into one litre (1dm3) of water. So how would you make up these solutions? 1. 1L 1M NaCl 2. 500mL 2M NaCl 3. 1L 1M H2SO4 4. 200ml 0.2M H2SO4 5. 2.5L 0.4M KOH 6. 300mL 1M MgCl2 7. 500mL 2M sucrose solution 8. 400mL 3M glucose solution Check with the DailyCalcs iPhone app http://itunes.apple.com/gb/app/dailycalcs- science-calculator/id353223512?mt=8 27 Glass of water from: http://openclipart.org/detail/132019/glass-of-water-by-gustavorezende
- 28. The rest of this presentation is on GoogleSlides, as I keep updating it. Here it is: http://i-biology.net/myp/intro- chemistry/the-mole-stoichiometry/ 28
- 29. For more resources. Please consider a donation to charity via Biology4Good. Click here for more information about Biology4Good charity donations. This is a Creative Commons presentation. It may be linked and embedded but not sold or re-hosted.
