Extra problem for 1st yr
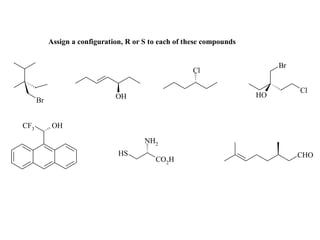
- 1. Assign a configuration, R or S to each of these compounds Br Cl Cl OH HO Br CF3 OH NH2 HS CHO CO2H
- 2. Problem: Compound I has two stereoisomers, but compound II and III exist as single compounds. Explain? + + Me N H Me N H Me N Et Cl Me Cl H I II III Problem: The following compound has only one chirality center, why then dose it have four stereoisomers Br
- 3. 1. KMnO4 HO A (chiral) + B (achiral) OH (R) Identify A & B 2. H2/Ni C (chiral) + D (achiral) Both C & D are C8H18, identify them. (R) 3. Two stereoisomers are obtained from the reaction of HBr with (3S, 4S)-4-bromo-3-methyl-1-pentene. one of the stereoisomer is optically active and the other is not. Explain? Me H + HBr Br H Me
- 4. Problem: Feist's acid (C6H6O4) is a cyclopropane based natural product and having two stereocenter. The compound is chiral. Based on this information predict its correct structure? CO2H CO2H Hint: CO2H HO2C CO2H CO2H X X HO2C CO2H HO2C CO2H right structure
- 5. Discuss the stereochemistry of the following compounds and mention about their optical activity with good diagrams. OH NHCO2Me O Ph Me O SMe O SMe N H
- 6. Problem: Which of the following are chiral and why? Me Me Me Me Me Me Me a(p) Me a(p) c a(p) Me Cl Me Me Cl Me Cl Cl Cl Me Cl Me Me Cl Me c a(p) a(c) a(p)
- 7. Problem: Draw all the isomers with mol. formula C 6H-12 that contain a cyclobutane ring, and comment on their chirality Hint: the base structure is dimethyl cyclobutane [1,2 (cis & trans) and 1,3 (cis & trans) also 1,1]; there also exist 1ethyl cyclobutane. Me Me Me Me Me Me
- 8. Problem: 2,5-dimethyl-1,1-cyclopentane dicarboxylic acid exist as two optically inactive isomers (A & B; differ in mp),Upon heating (mono decarboxylation A yields two 2,5-dimethylcyclopentanecarboxylic acid and B yields one. Assign structures of A & B. HO2C CO2H Hint: CO2H CO2H heat A + Me Me CO2H CO2H HO2C CO2H heat or B same A & B are diastereomers
- 9. Indicate wheather each of the following pairs of compounds are identical or enantiomers, diastereomers or constitutional isomers. H Me Me Et a. Et CH2OH and H Et b. HO H HO H Me CH2OH and H Cl H Cl Et Me Me Me HO H H OH c. and d. and Cl H Cl Cl H Me Me Cl Cl H Cl e. H H H Cl Cl H H Cl and f. and Cl Cl Cl H H Cl Cl H Br H CH2Cl Et H Br g. h. and Et Me and Me CH2Cl H Br H H Br H i. H j. and and Br H Br
- 10. Problem: Which of the following compounds are resolvable, and which are non resolvable?Which are truly meso? a) cis-1,2-cyclohexane diol; b) trans-1,2-cyclohexane diol; c) cis-1,3-cyclohexane diol; d) trans-1,3-cyclohexane diol; e) cis-1,4-cyclohexane diol; f) trans-1,4-cyclohexane diol. Hint: OH OH OH OH OH OH OH OH trans (resolvable) Non resolvable (easily interconvertible by flipping) OH OH OH HO OH OH cis (meso) trans (resolvable) OH OH HO OH HO OH achiral (absence of chirality center)
- 11. Problem: On treatment with the aromatic base pyridine, racemic 1,2-dibromo-1,2-diphenyl ethane loses HBr to yield trans-1-bromo-1,2-diphenyl ethane; In contrast the meso dibromide loses Br 2 to yield trans-1,2-diphenyl ethene. Suggets a mechanism? Hnit: Ph Br Ph Br Ph H Br H H H Ph Br2 loss is not favored Ph H Br H Br Br Ph Racemic H Ph -HBr Ph H Br Ph Br Ph Ph Ph Br Br PhH Ph H Br Ph Br H H -Br2 H Br Ph Ph H Br Ph Br meso
- 12. Problem: Reduction of 4-t-butyl cyclohexanone with LiAlH 4 gives exclusively trans alcohol; whereas with lithiumtri-sec butylborohydride yields cis`alcohol exclusively? explain. Hnit: OH H Li-sBu3BH O LiAlH4 H OH H Al H H B H H
- 13. It is more difficult to form an acetal of compound A than B? O OH O HO O H+ A O OH O HO O H+ A R R R R + Hint: H+ O O OH+ OH R H+ R R O -H2O R OH OH HO OH R R O O R O + O R H HO OH HO OH O O
- 14. Problem: Which of these two compounds would form an epoxide on treatment with base? OH OH Br Br H H A B Hint: Me OH Me OH Br H Br H A B
- 15. Problem: Only one of the diastereomeric bromides shown here eliminates to give alkene A. Why? Neither bromide gives alkene B. Why not? O O O O Base O Br O A O O Base Alkene B No alkene A Br Hint: O O O O Br Br
- 16. N + CH3I N CH3 + I Explain the relative rate Me N Me N Me N Me N 2.27 0.47 0.042 1.00
- 17. Explain the reaction sequences with proper explanation. H Ph heat Ph H heat Ph HO2C HO2C HO2C
- 18. Identify the missing products in the following reaction sequence. O a) MgBr 175oC ? ? H b) KH, 60oC O H O
- 19. Each of the following reactions involves one or more concerted steps that take place in accordance with theWoodward-Hoffmann rules. In each case, prdeict exactly what is happening, with stereochemistry? 1. 25oC O Hint: (ring junction cis-beta) O 100oC light 25oC 2. cis-beta trans H H 140oC 220oC 3. H H
- 20. Problem: Account for the difference in conditions required to bring about the following transformations Me Me Me Me 176oC H Me Me Me Me H Me H Me 400oC Me Me H Problem: Give stereochemical structures of A and B and tell exactly what process is taking place in each reaction 100oC cis, cis, cis-cycloocta-1,3,5-triene A (C8H10) A+ MeO2C CO2Me B (C14H16O4) heat B Cyclobutene + dimethylphthalate
- 21. Predict the products in the following reactions; O 1. OH KH H+ ? ? THF, heat 2. MeCOCl LDA heat OH Ph OH ? ? ? Et3N H+ Ph O Ireland-Claisen rearrangement 3. Suggest a mechanism for this reaction? Me O + O O O H CO2Me CO2Me
- 22. Each of the following transformations is believed to proceed by the indicated sequence of concerted reactions. Show just what each step involves, and give structures of each intermediates 1. H H R heat heat R A R R H H Both electrocyclic closure 2. 200oC 260oC B C 1,5-H shift, electrocyclic opening. Me H H Et Me 170oC 170oC 3. D Et H H electrocyclic opening, electrocyclic closure
- 23. The deuterium scrambling has been accounted for on the basis ofintramolecular Diels-Alder and retro DA reactions. Show how might this occur? heat D D D Hint: look for an intermediate that is symmetrical except for the presence of deuterium.
- 24. ? + N2 ? ? CO2- O ?
- 25. Which alkyl halide would you expect to be more reactive in an SN2/SN1 reaction with a given nucleophile? a. Br and I e. Br Br b. Cl and and O Cl f. and Br c. Br Br Br and g. Br and d. and Br Br Br
- 26. Problem: When cis-1-bromo-4-methyl cyclohexane undergoes an SN2 reaction, only trans-4-methylcyclohexanolis obtained, where as under SN1 reaction condition both cis and trans product is obtained. Explain Hint: Br Br Me OH- Me Me OH SN2 OH- trans cis Br OH H 2O + Me Me Me OH Me SN1 cis trans cis Problem: Which of the following will react faster in an SN1 reaction? But Br But Br
- 27. Problem: Which of the following compounds would you expect to be more reactive in an SN2 reaction? Br Br Hint: Br Me Me Me Br Me A B A B Ans: A Problem: Which of the following compounds would you expect to be more reactive in an SN2 reaction? But OTs OTs Ans: A A B
- 28. NH2 NaNO2, H2O CHO Problem1 OH H2SO4 NaNO2, H2O O H2SO4 Problem 2 O AgNO3 HO CHO Br Problem 3: Kinetic measurements reveal that solvolytic displacement of sulfonate is about 5 X 105 faster for 3B than for 3A OSO2Ar OSO2Ar O O 3A 3B
- 29. Q. O X X X X O O Relative rate 1.0 0.014 0.14 4.85 x 10 4 Explain Transannular participation of ether oxygen O O O . I II III . III is more favorable than either I or II Unfavorable polar effect of the C-O bond
- 30. OH OPh NMe2 DEAD, TPP DEAD, TPP NMe2 NMe2 OH PhOH PhOH A B Both A and B gave the same priduct when subjected to Mitsunobu conditions with phenol as nucleophile H H OH DEAD, TPP NO2 NO2 H Explain the reaction
- 31. PhOH Me OPh + N Me NMe2 common intermediate OH OP+Ph3 OP+Ph3 TPP NO2 DEAD NO2 NO2 C NO2 H
- 32. O H2O HO CHO Br
- 33. O O O Br + hemiacetal C HO + O HO CHO
- 34. Q. Describe the stereochemistry of the products of these reactions. Cl O LiAlH4 S (a) S (_) + - forms, inversion takes place (SN2) at C-atom containing Cl, forms cis-fused product OH O S base (b) HS enantiomerically pure Both are intramolecular SN2
- 35. H N O Product OTs OTs KOH/H2S Product
- 36. OH O (a) + N Ph N Ph H OTs OTs KOH (b) H2S S HS SN 2 SH OTs S S OTs
- 37. Q. Suggest a mechanism for the following reaction O O H2O O Ph Ph Cl N Ph N CH3CN, heat H Ph O O O O O Cl Ph N Ph N Ph N O O Ph O Ph Ph OH O O Ph Ph N H O
- 38. Predict the final product I2, Ph3P MeO CH2OH N N H NBS, TPP MeO CH2OH
- 39. For each of the compounds A through H indicate the number of gauche butane interactions present in the most stable chair conformation.
- 40. Me Me OH OH HO Br OH O O H
- 41. But But OH HO Br OH OH O O H