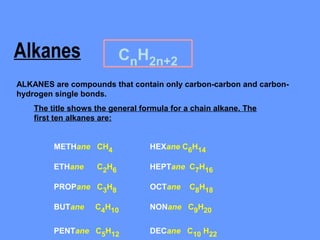
Alkanes CnH2n+2 Formula & Naming Rules
- 1. Alkanes CnH2n+2 ALKANES are compounds that contain only carbon-carbon and carbon- hydrogen single bonds. The title shows the general formula for a chain alkane. The first ten alkanes are: METHane CH4 HEXane C6H14 ETHane C2H6 HEPTane C7H16 PROPane C3H8 OCTane C8H18 BUTane C4H10 NONane C9H20 PENTane C5H12 DECane C10 H22
- 2. Lewis dot vs condensed formulas: propane. H H H H C C C H CH3CH2CH3 H H H Terminal carbons condense to CH3 with the hydrogens usually to the right of the carbon. Interior carbons condense to CH2.
- 3. Branching A branch or substituents on a chain may be condensed into the chain usually after the carbon from which it branches. CH3CH(CH3)CHClCH2CH3 H HCH H H H H H C C C C C H H H Cl H H
- 4. NOMENCLATURE Alkyl Groups H H H C H H C or CH3 H H An alkyl group is an alkane with one hydrogen atom removed. It is named by replacing the -ane of the alkane name with -yl. e.g. Methane becomes a methyl group.
- 5. For PROPANE, removal of a hydrogen generates two different propyl groups depending on whether an end or center H is removed. CH3 CH2 CH3 CH3CH2CH2 CH3CH CH3 n-propyl isopropyl
- 6. For BUTANE, gives two butyl groups depending on whether an end (1o) or interior (2o) H is removed. CH3 CH2 CH2 CH3 CH3 CH2 CH2 CH2 CH3 CH CH2 CH3 n-butyl sec-butyl
- 7. ISOBUTANE gives two butyl groups depending on whether a 10 or 30 H is removed. CH3 CH3 CH CH3 CH3 CH3 1o 3o CH3 CH CH2 CH3 C CH3 isobutyl tert-butyl
- 8. NOMENCLATURE 1. The base or parent name for an alkane is determined by the longest chain of carbon atoms in the formula. Note: the longest chain may bend and twist. It is seldom horizontal! – Any carbon groups not part of the base chain are called branches or substituents. – These carbon groups are also called alkyl groups. A CH3 CH2CH2CH CH2CH3 B CH3 CH2 CH3CH2CH2 CH2 CH3 CH3CH2CH CH2CH CH3 A. 7 carbons B. 8 carbons
- 9. 2. Number the carbon atoms in the chain starting from the end with the first branch. If both branches are equally from the ends, continue until a point of difference occurs. 1 CH3 6 7 8 CH3 2 CH2 CH2CH2CH3 4 5 CH2CH2CH CH2CH3 CH3CH CH2CH CH2CH3 3 2 1 3 4 5 6 7 6 CH2 this branch would be on C-4 7 CH3 if you started at correct C-8
- 10. 3. Prefix the branches/substituents in alphabetical order before the base/stem name (longest chain). Halogens usually come first. - Indicate the position of the branch on the main chain by prefixing its name with the carbon number to which it is attached. Separate numbers and letters with a hyphen. CH3 CH2 CH3 CH3 CH2 CH CH2 CH CH3 4-ethyl-2-methylhexane
- 11. - Hyphenated and number prefixes are not considered when alphabetizing groups. 5-sec-butyl-4-isopropylnonane CH3 CH3 CH CH2 CH3 CH CH CH CH2 CH2 CH2 CH3 CH3 CH2 CH2 CH3
- 12. - When a branch/substituents occurs more than once, prefix the name with di-, tri-, tetra-, etc. Then prefix the number to the name with a separate number for each occurrence. Separate numbers with commas. CH3 CH2CH3 CH3CH CH CH2CH CH2CH3 CH3 5-ethyl-2,3-dimethylheptane NOTE: ethyl > dimethyl
- 13. CH3 CH3CH CH3 CH2 CH2 CH3C CH2CH2C CH2CH3 CH3 CH2CH2CH2CH3 6-isobutyl-6-ethyl-3,3-dimethyldecane
- 14. Alkenes and Alkynes • ALKENES are compounds that have carbon-carbon double bond framework. With the general formula of, CnH2n. Simplest alkene: ethene (ethylene) C2H4 • ALKYNES have one or more carbon-carbon triple bonds. Simplest alkyne: ethyne (acetylene) C2H2
- 15. NOMENCLATURE Base name from longest chain containing the multiple bond. Change -ane to -ene or -yne. Number from the end that will give the first carbon of the multiple bond the lower number. Prefix the name with the number of the first multiple bond carbon. Prefix branch/substituent names as for alkanes. CH3 CH2 CH3 3-ethyl-6-methyl-3-heptene CH3 CH CH2 CH C CH2 CH3 2-bromo-3-hexyne CH3CH C C CH2CH3 Br
- 16. Cyclic alkenes are named like cyclic alkanes. Prefix name with cyclo-. Numbering MUST start at one end of the double bond and go THROUGH the bond. Substituents must have the lower possible numbers. CH2 CH3 Cl CH CH 5-chloro-3-methylcyclohexene CH2 CH CH
- 17. ALCOHOLS AND PHENOLS Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°) depending on the number of carbon groups bonded to the hydroxyl bearing carbon. CH3CH2OH Primary: ONE R group on the carbon attached to -OH CH3CH CH3 Secondary: OH TWO R groups on the carbon attached to -OH CH3 Tertiary: THREE R groups on the carbon attached to -OH CH3 C CH3 OH
- 18. ALCOHOLS AND PHENOLS NOMENCLATURE Alcohols are classified as primary (1o), secondary (2o), or tertiary, depending on the numbers of carbon substituents bonded to the hydroxyl-bearing carbon: STEP 1 Select the longest chain containing the hydroxyl and replace the –e ending of the corresponding parent with –ol STEP 2 Number the carbons of the parent chain beginning at the end nearer the hydroxyl STEP 3 Number all substituents according to their position on the chain, and write the name listing the substituents in alphabetical order.
- 19. ETHERS NOMENCLATURE Common names for ethers consist of the names of the two groups attached to the O listed in alphabetical order (or size) and followed by ‘ether’. Each part is a separate word O CH3 CH3CH – O – CH3 Isopropyl methyl ether Ethyl phenyl ether The IUPAC names for ethers are based on the alkane name of the longest chain attached to the O. The shorter chain is named as an alkoxy substituent. (alkane with the ane replaced by oxy, eg. CH3CH2O = ethoxy). The longest chain will be the parent structure (-ane). CH3CH2CH2CH2CH2-O-CH3 1-methoxypentane methyl pentyl ether CH3CH2-O-CH2CH2CH2CH3 1-ethoxybutane ethyl butyl ether
- 20. THIOLS Thiols (mercaptans), R-SH, are sulfur analogs of alcohols, and sulfides, RSR’, are sulfur analogs of ether. The SH group is variously called the thiol group, the mercaptan group, or the sulfuhydryl group. NOMENCLATURE Name based on the longest alkane chain with the suffix position indicated by a number. 3D CH3 CH3 – CH - CH2 – CH2 SH 3-methyl-1-butanethiol
- 21. CARBONYL COMPOUNDS O • contains the carbonyl group, C=O R C R Aldehydes: O R may be hydrogen usually a carbon containing group R C H RCHO O Ketones: R contains carbon RC R RCOR
- 22. Aldehydes NOMENCLATURE STEP 1 Select the longest carbon chain containing the carbonyl carbon. STEP 2 The -e ending of the parent alkane name is replaced by the suffix -al. STEP 3 The carbonyl carbon is always numbered “1.” (It is not necessary to include the number in the name.) STEP 4 Name the substituents attached to the chain in the usual way. O O 4 O 3 2 CH3CH CH3CH2CH CH3CH CH2CH2CH Ethanal Propanal H2C 5 CH 1 (Acetaldehyde) (Propionaldehyde) 3 6 4-methylhexanal
- 23. KETONES NOMENCLATURE STEP 1 Naming the two alkyl groups attached to the carbonyl in a) alphabetical order or b) by increasing size. O Methyl ethyl ketone or CH3 C CH2 CH3 Ethyl methyl ketone STEP 2 Longest chain with the C=O. The corresponding alkane is (-e) is replaced with -one STEP 3 The position of C=O by number of chain is indicated. Cl O 4-chloro-2-pentanone CH3CH CH2C CH3
- 24. CARBOXYLIC ACIDS O O C OH or -CO2H or -COOH S OH or -SO3H carboxyl group O sulfonic acid group O R = may be H, R C OH alkyl,or aromatic
- 25. NOMENCLATURE Start numbering at the -COOH carbon. Replace the -e of the alkane name with oic acid. CH3 O CH3 CH CH2 C OH 4 3 2 1 3-methylbutanoic acid
- 26. The Acid Derivatives AMIDES O R2 R1, R2, R3 may be R1 C N H, alkyl, aryl R3 ESTERS O R2 should not R1 C O R2 be H
- 27. ESTERS Esters are derivatives of an ACID and an ALCOHOL that can also be hydrolyzed to these parent compounds. ESTER LINKAGE O R = may be R C OR alkyl,or aromatic
- 28. NOMENCLATURE Name the alkyl group attached to the oxygen atom first. (Alcohol part of the ester). Base name for the acid part of the structure from the longest chain ending in the C=O. Change the oic acid of the acid name to oate and add this to the name in 1 as a second word. O CH3CH2C O CH2CH3 ethyl propanoate
- 29. AMINES NOMENCLATURE • Primary amines are named in systematic (IUPAC) nomenclature by replacing the -e of the corresponding parent alkane with -amine • In common nomenclature they are named as alkylamines • Simple secondary and tertiary amines are named in common nomenclature by designating the organic groups separately in front of the word amine • In systematic nomenclature, the smaller groups on the amine nitrogen are designated as substituents and given the locant N
- 30. AMIDES NOMENCLATURE STEP 1 Names are derived from the acid. STEP 2 Remove –ic acid (common) or –oic acid (IUPAC) and replace with –amide. STEP 3 Nitrogen substituents are prefixed to the name and indicated by N. O O CH3C NH2 CH3CH2C NH CH3 ethanamide or acetamide N-methylpropanamide
