Electron Orbitals Physical Science Lesson PowerPoint
- 1. • The number of valence electrons determines the group placement of an element on the periodic table. Copyright © 2010 Ryan P. Murphy
- 3. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. Copyright © 2010 Ryan P. Murphy
- 4. -Nice neat notes that are legible and use indents when appropriate. -Example of indent. -Skip a line between topics - -Make visuals clear and well drawn. Label please. Neutron Proton Electron
- 5. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. • BLACK SLIDE: Pay attention, follow directions, complete projects as described and answer required questions neatly. Copyright © 2010 Ryan P. Murphy
- 8. John Dalton’s Atomic Theories -All matter is composed of _________. -Atoms cannot be made or __________. -All atoms of the same element are _________. -Different elements have different types of _____. -Chemical reactions occur when atoms are ____________. -Compounds are formed from atoms of the elements.
- 9. John Dalton’s Atomic Theories -All matter is composed of _________. -Atoms cannot be made or __________. -All atoms of the same element are _________. -Different elements have different types of _____. -Chemical reactions occur when atoms are ____________. -Compounds are formed from atoms of the elements.
- 10. John Dalton’s Atomic Theories -All matter is composed of atoms. -Atoms cannot be made or __________. -All atoms of the same element are _________. -Different elements have different types of _____. -Chemical reactions occur when atoms are ____________. -Compounds are formed from atoms of the elements.
- 11. John Dalton’s Atomic Theories -All matter is composed of atoms. -Atoms cannot be made or __________. -All atoms of the same element are _________. -Different elements have different types of _____. -Chemical reactions occur when atoms are ____________. -Compounds are formed from atoms of the elements.
- 12. John Dalton’s Atomic Theories -All matter is composed of atoms. -Atoms cannot be made or destroyed. -All atoms of the same element are _________. -Different elements have different types of _____. -Chemical reactions occur when atoms are ____________. -Compounds are formed from atoms of the elements.
- 13. John Dalton’s Atomic Theories -All matter is composed of atoms. -Atoms cannot be made or destroyed. -All atoms of the same element are _________. -Different elements have different types of _____. -Chemical reactions occur when atoms are ____________. -Compounds are formed from atoms of the elements.
- 14. John Dalton’s Atomic Theories -All matter is composed of atoms. -Atoms cannot be made or destroyed. -All atoms of the same element are identical. -Different elements have different types of _____. -Chemical reactions occur when atoms are ____________. -Compounds are formed from atoms of the elements.
- 15. John Dalton’s Atomic Theories -All matter is composed of atoms. -Atoms cannot be made or destroyed. -All atoms of the same element are identical. -Different elements have different types of _____. -Chemical reactions occur when atoms are ____________. -Compounds are formed from atoms of the elements.
- 16. John Dalton’s Atomic Theories -All matter is composed of atoms. -Atoms cannot be made or destroyed. -All atoms of the same element are identical. -Different elements have different types of atoms. -Chemical reactions occur when atoms are ____________. -Compounds are formed from atoms of the elements.
- 17. John Dalton’s Atomic Theories -All matter is composed of atoms. -Atoms cannot be made or destroyed. -All atoms of the same element are identical. -Different elements have different types of atoms. -Chemical reactions occur when atoms are ____________. -Compounds are formed from atoms of the elements.
- 18. John Dalton’s Atomic Theories -All matter is composed of atoms. -Atoms cannot be made or destroyed. -All atoms of the same element are identical. -Different elements have different types of atoms. -Chemical reactions occur when atoms are rearranged. -Compounds are formed from atoms of the elements.
- 19. John Dalton’s Atomic Theories -All matter is composed of atoms. -Atoms cannot be made or destroyed. -All atoms of the same element are identical. -Different elements have different types of atoms. -Chemical reactions occur when atoms are rearranged. -Compounds are formed from atoms of the elements.
- 21. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. D.) Different elements have the same type of atoms. E.) Chemical reactions occur when atoms are rearranged. F.) Compounds are formed from atoms of the constituent elements. Copyright © 2010 Ryan P. Murphy
- 22. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. D.) Different elements have the same type of atoms. E.) Chemical reactions occur when atoms are rearranged. F.) Compounds are formed from atoms of the constituent elements. Copyright © 2010 Ryan P. Murphy
- 23. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. D.) Different elements have different types of atoms. E.) Chemical reactions occur when atoms are rearranged. F.) Compounds are formed from atoms of the constituent elements. Copyright © 2010 Ryan P. Murphy
- 24. “One more time.”
- 25. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are different. D.) Different elements have different types of atoms. E.) Chemical reactions occur when atoms are rearranged. F.) Compounds are formed from atoms of the constituent elements. Copyright © 2010 Ryan P. Murphy
- 26. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are different. D.) Different elements have different types of atoms. E.) Chemical reactions occur when atoms are rearranged. F.) Compounds are formed from atoms of the constituent elements. Copyright © 2010 Ryan P. Murphy
- 27. • Which is not one of John Daltons Atomic Theories? A.) All matter is composed of atoms. B.) Atoms cannot be made or destroyed. C.) All atoms of the same element are identical. D.) Different elements have different types of atoms. E.) Chemical reactions occur when atoms are rearranged. F.) Compounds are formed from atoms of the constituent elements. Copyright © 2010 Ryan P. Murphy
- 28. “I was one of the first people to document color blindness.” Learn more: http://www.biography.com/ people/john-dalton-9265201
- 29. • The Greek root for the word atom, "atomon," means "that which cannot be divided." Copyright © 2010 Ryan P. Murphy
- 30. • The Greek root for the word atom, "atomon," means "that which cannot be divided." –But atoms can be divided! Copyright © 2010 Ryan P. Murphy
- 31. • The Greek root for the word atom, "atomon," means "that which cannot be divided." –But atoms can be divided! –But not easily on Earth. Copyright © 2010 Ryan P. Murphy
- 32. • The Greek root for the word atom, "atomon," means "that which cannot be divided." –But atoms can be divided! –But not easily on Earth. Copyright © 2010 Ryan P. Murphy
- 33. Each Element is made up of one kind of atom. The number of Protons and Electrons. Atoms are arranged on The Periodic Table of Elements.
- 34. Each Element is made up of one kind of atom. The number of Protons and Electrons. Atoms are arranged on The Periodic Table of Elements.
- 37. • Hydrogen – 1 proton – Has an atomic mass of 1. Copyright © 2010 Ryan P. Murphy
- 38. • Hydrogen – 1 proton – Has an atomic mass of 1. Copyright © 2010 Ryan P. Murphy
- 39. • Hydrogen – 1 proton – Has an atomic mass of 1. • Helium – 2 protons and 2 neutrons – Has an atomic mass of 4. Copyright © 2010 Ryan P. Murphy
- 40. • Hydrogen – 1 proton – Has an atomic mass of 1. • Helium – 2 protons and 2 neutrons – Has an atomic mass of 4. Copyright © 2010 Ryan P. Murphy
- 41. Atomic Mass = AMU Atomic Mass Units, The number of protons, neutrons, and electrons. Copyright © 2010 Ryan P. Murphy
- 42. • Review! To find # of protons and electrons Copyright © 2010 Ryan P. Murphy
- 43. • Review! To find # of protons and electrons – It is the atomic number, or count the P+ or E-. Copyright © 2010 Ryan P. Murphy
- 44. • Review! To find # of protons and electrons – It is the atomic number, or count the P+ or E-. Copyright © 2010 Ryan P. Murphy
- 45. • Review! To find # of protons and electrons – It is the atomic number. – What is this atoms Atomic number? Copyright © 2010 Ryan P. Murphy
- 46. • Answer! Copyright © 2010 Ryan P. Murphy
- 47. • Answer! Count the 11 Protons or 11 Electrons Copyright © 2010 Ryan P. Murphy
- 48. • Answer! Atomic Number 11. – What element is #11 Copyright © 2010 Ryan P. Murphy
- 49. • Answer! Atomic Number 11. – What element is #11 Sodium Copyright © 2010 Ryan P. Murphy
- 50. • Review! To find # of neutrons Copyright © 2010 Ryan P. Murphy
- 51. • Review! To find # of neutrons – Subtract the atomic number from the atomic mass to determine the difference. Copyright © 2010 Ryan P. Murphy
- 52. • Review! To find # of neutrons – Subtract the atomic number from the atomic mass to determine the difference. – How many neutrons does Lithium have? Copyright © 2010 Ryan P. Murphy
- 53. • Review! To find # of neutrons – Subtract the atomic number from the atomic mass to determine the difference. – How many neutrons does Lithium have? Atomic Mass Copyright © 2010 Ryan P. Murphy
- 54. • Review! To find # of neutrons – Subtract the atomic number from the atomic mass to determine the difference. – How many neutrons does Lithium have? Atomic Mass Atomic Number Copyright © 2010 Ryan P. Murphy
- 55. • Review! To find # of neutrons – Subtract the atomic number from the atomic mass to determine the difference. – How many neutrons does Lithium have? Atomic Mass Atomic Number 6.94 amu – 3 = Copyright © 2010 Ryan P. Murphy
- 56. • Answer! 6.94 – 3 = 3.94 Atomic Mass Atomic Number 6.94 amu – 3 = Copyright © 2010 Ryan P. Murphy
- 57. • Answer! 6.94 – 3 = 3.94 or 4 neutrons Atomic Mass Atomic Number 6.94 amu – 3 = Copyright © 2010 Ryan P. Murphy
- 58. • Mini-Periodic Table Available Sheet
- 59. • Mini-Periodic Table Available Sheet
- 60. • Activity! Please create the table on the next slide, 4 down x 10 across. – Use the Periodic Table of Elements. Copyright © 2010 Ryan P. Murphy
- 61. H He Copyright © 2010 Ryan P. Murphy
- 62. H He Li Na K Copyright © 2010 Ryan P. Murphy
- 63. H He Li Ne Na Ar K Kr Copyright © 2010 Ryan P. Murphy
- 64. H He Li Be B C N O F Ne Na Ar K Kr Copyright © 2010 Ryan P. Murphy
- 65. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Kr Copyright © 2010 Ryan P. Murphy
- 66. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Copyright © 2010 Ryan P. Murphy
- 67. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Copyright © 2010 Ryan P. Murphy H
- 68. New Area of Focus: Electron Orbitals Copyright © 2010 Ryan P. Murphy
- 69. • Niels Bohr (1915): Student to Ernest Rutherford. – The Bohr model is a simplified picture of an atom. We will spend a lot of time learning this, and then…. – The correct theory of the atom… • (Quantum Mechanics) • More Difficult
- 70. • Niels Bohr (1915): Student to Ernest Rutherford. – The Bohr model is a simplified picture of an atom. We will spend a lot of time learning this, and then…. – The correct theory of the atom… • (Quantum Mechanics) • More Difficult Learn more http://chemed.chem.pu rdue.edu/genchem/hist ory/bohr.html “Hey, I suggested the idea that electrons move from one energy level to another in large steps.”.-Quantum…. Learn more: http://en.wikipedia.org/wi ki/Niels_Bohr
- 71. • Video Link! Electron Orbitals – http://www.youtube.com/watch?v=rNM21emk MJo
- 72. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy
- 73. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy How many valence electrons?
- 74. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy
- 75. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1
- 76. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1
- 77. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2
- 78. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2
- 79. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2 1
- 80. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2 1
- 81. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2 1 4
- 82. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2 1 4
- 83. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2 1 4 5
- 84. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2 1 4 5
- 85. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2 1 4 5 1
- 86. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2 1 4 5 1 Total Electrons
- 87. Valence electrons: Electrons in the outer most shell. Copyright © 2010 Ryan P. Murphy 1 2 1 4 5 1 Total Electrons
- 88. • Activity! Quiz Wiz: Name the atom based on the electron orbitals / atomic number. – Also tell me how many valence the atom has? Copyright © 2010 Ryan P. Murphy
- 89. • Answers! Quiz Wiz: The answer will be given after each questions instead of at the end. Copyright © 2010 Ryan P. Murphy
- 90. • Answers! Quiz Wiz: The answer will be given after each questions instead of at the end. Copyright © 2010 Ryan P. Murphy “This Quiz Wiz will require you to work in table groups.”
- 111. 2+8+18+32=60
- 112. 2+8+18+32=60
- 113. 2+8+18+32=60
- 114. 2+8+18+32=60
- 115. 2+8+18+32=60
- 116. 2+8+18+32=60
- 117. • Bonus: What is this, and what characters have worn it. Do you know the order. Copyright © 2010 Ryan P. Murphy
- 118. • Bonus: Lost by the Dark Lord Sauron, Copyright © 2010 Ryan P. Murphy
- 119. • Bonus: Lost by the Dark Lord Sauron, Found by Smeagol / Gollum – Lost in cave, Copyright © 2010 Ryan P. Murphy
- 120. • Bonus: Lost by the Dark Lord Sauron, Found by Smeagol / Gollum – Lost in cave, Found by Bilbo Baggins, Copyright © 2010 Ryan P. Murphy
- 121. • Bonus: Lost by the Dark Lord Sauron, Found by Smeagol / Gollum – Lost in cave, Found by Bilbo Baggins, and given to Frodo Baggins. LOTR Trilogy & Hobbit. Copyright © 2010 Ryan P. Murphy
- 126. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up.
- 127. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up.
- 128. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up. This is really difficult and I’m going to quit as soon as I don’t know it. I’m going to check out completely or create issues for those choosing A.
- 129. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up. This is really difficult and I’m going to quit as soon as I don’t know it. I’m going to check out completely or create issues for those choosing A.
- 130. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up. This is really difficult and I’m going to quit as soon as I don’t know it. I’m going to check out completely or create issues for those choosing A.
- 131. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up. This is really difficult and I’m going to quit as soon as I don’t know it. I’m going to check out completely or create issues for those choosing A.
- 132. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up. This is really difficult and I’m going to quit as soon as I don’t know it. I’m going to check out completely or create issues for those choosing A.
- 133. • Electron Orbitals Available Sheet
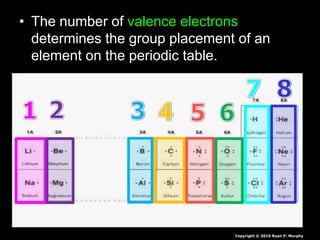
- 134. The number of valence electrons determines the group placement of an element on the periodic table. Copyright © 2010 Ryan P. Murphy
- 135. The number of valence electrons determines the group placement of an element on the periodic table. Copyright © 2010 Ryan P. Murphy
- 136. The number of valence electrons determines the group placement of an element on the periodic table. Copyright © 2010 Ryan P. Murphy
- 137. The number of valence electrons determines the group placement of an element on the periodic table. Copyright © 2010 Ryan P. Murphy
- 138. The number of valence electrons determines the group placement of an element on the periodic table. Copyright © 2010 Ryan P. Murphy
- 139. The number of valence electrons determines the group placement of an element on the periodic table. Copyright © 2010 Ryan P. Murphy
- 140. The number of valence electrons determines the group placement of an element on the periodic table. Copyright © 2010 Ryan P. Murphy
- 141. The number of valence electrons determines the group placement of an element on the periodic table. Copyright © 2010 Ryan P. Murphy
- 142. The number of valence electrons determines the group placement of an element on the periodic table. Copyright © 2010 Ryan P. Murphy
- 143. • Electron Orbitals Available Sheet
- 144. The 1, 2, 3, 4 electron shell can hold… 2, 8, 18, 32 electrons
- 146. The 1, 2, 3, 4 electron shell can hold…
- 147. The 1, 2, 3, 4 electron shell can hold… 2, 8, 8, 2 electrons
- 148. The 1, 2, 3, 4 electron shell can hold… 2, 8, 8, 2 electrons For the first 20 elements the pattern is 2, 8, 8, 2
- 149. The 1, 2, 3, 4 electron shell can hold… 2, 8, 8, 2 electrons For the first 20 elements the pattern is 2, 8, 8, 2
- 151. Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones.
- 164. Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones.
- 181. Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones.
- 193. “If you have sidewalk chalk bring it for the next activity.”
- 194. • Activity! Going outside and creating the atom Nitrogen #7 Copyright © 2010 Ryan P. Murphy
- 195. • Activity! Going outside and creating the atom Nitrogen #7 – Students need to be protons, neutrons, and electrons in the correct orbitals. Copyright © 2010 Ryan P. Murphy
- 196. • Activity! Going outside and creating the atom Nitrogen #7 – Students need to be protons, neutrons, and electrons in the correct orbitals. • Boys neutrons, girls protons in nucleus? Copyright © 2010 Ryan P. Murphy
- 197. • Activity! Going outside and creating the atom Nitrogen #7 – Students need to be protons, neutrons, and electrons in the correct orbitals. • Boys neutrons, girls protons in nucleus? – Bring your Periodic Table because teacher will require you to build a few more atoms. Copyright © 2010 Ryan P. Murphy “Okay Nucleons.” “Do you have your periodic table and sidewalk chalk to some elements of my choosing after we build nitrogen?”
- 199. Most of the transition metals… 2, 8, 18, Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy 2 8 18
- 200. Most of the transition metals… 2, 8, 18, Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy 2 8 18 The transition metals are able to put up to 32 electrons in their second-to-last shell
- 201. Most of the transition metals… 2, 8, 18, 32, 32, 18, 2 Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy 2 8 18 32 32 18 The transition metals are able to put up to 32 electrons in their second-to-last shell
- 202. 2
- 203. • After the second orbit or shell is filled, things start to get complicated. The third shell fills until it gets to 8, and then the fourth shell starts adding electrons until it too has 8 electrons. Then the third shell fills until it gets to 18. 2 8
- 204. • After the second orbit or shell is filled, things start to get complicated. The third shell fills until it gets to 8, and then the fourth shell starts adding electrons until it too has 8 electrons. Then the third shell fills until it gets to 18. 2 8 8
- 205. • After the second orbit or shell is filled, things start to get complicated. The third shell fills until it gets to 8, and then the fourth shell starts adding electrons until it too has 8 electrons. Then the third shell fills until it gets to 18. 2 8 8 8
- 206. • After the second orbit or shell is filled, things start to get complicated. The third shell fills until it gets to 8, and then the fourth shell starts adding electrons until it too has 8 electrons. Then the third shell fills until it gets to 18. 2 8 18 8
- 207. • Electron Orbitals Available Sheet
- 208. Copyright © 2010 Ryan P. Murphy
- 209. • 2 electrons fill the first level Copyright © 2010 Ryan P. Murphy
- 210. • 2 electrons fill the first level Copyright © 2010 Ryan P. Murphy
- 211. • 2 electrons fill the first level • 8 electrons fill the second level, ring, or shell. Copyright © 2010 Ryan P. Murphy
- 212. • 2 electrons fill the first level • 8 electrons fill the second level, ring, or shell. Copyright © 2010 Ryan P. Murphy
- 213. • 2 electrons fill the first level • 8 electrons fill the second level, ring, or shell, and then… Copyright © 2010 Ryan P. Murphy
- 214. • 2 electrons fill the first level • 8 electrons fill the second level, ring, or shell, and then… Copyright © 2010 Ryan P. Murphy
- 215. • Electron Orbitals Available Sheet
- 216. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 217. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 218. • How many electrons are in the first three energy levels?
- 219. • How many electrons are in the first three energy levels?
- 220. • How many electrons are in the first three energy levels?
- 221. • How many electrons are in the first three energy levels?
- 222. • How many electrons are in the first three energy levels?
- 224. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 225. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 226. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 227. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 228. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 229. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 230. • How many electrons are in the first three energy levels? Copyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. MurphyCopyright © 2010 Ryan P. Murphy
- 231. Not Smart Board Activity! Arrange the electrons onto the Fluorine atom below #9 9 Protons Electrons
- 232. Not Smart Board Activity! Arrange the electrons onto the Fluorine atom below #9 9 Protons Electrons
- 233. Not Smart Board Activity! Arrange the electrons onto the Fluorine atom below #9 9 Protons Electrons
- 234. Not Smart Board Activity! Arrange the electrons onto the Fluorine atom below #9 Answer! 9 Protons Electrons
- 235. Not Smart Board Activity! Arrange the electrons onto the Fluorine atom below #9 Answer! 9 Protons Electrons 2
- 236. Not Smart Board Activity! Arrange the electrons onto the Fluorine atom below #9 Answer! 9 Protons Electrons 2 7
- 237. Not Smart Board Activity! Arrange the electrons onto the Fluorine atom below #9 Answer! 9 Protons Electrons 2 7 “I want one more.”
- 238. Not Smart Board Activity! Arrange the electrons onto the Sulfur atom below #16 16 Protons Electrons
- 239. Not Smart Board Activity! Arrange the electrons onto the Sulfur atom below #16 16 Protons Electrons
- 240. Not Smart Board Activity! Arrange the electrons onto the Sulfur atom below #16 16 Protons Electrons
- 241. Not Smart Board Activity! Arrange the electrons onto the Sulfur atom below #16 16 Protons Electrons
- 253. Not Smart Board Activity! Arrange the electrons onto the Potassium atom below #19 19 Protons Electrons
- 254. 19 Protons Electrons
- 255. 19 Protons Electrons
- 260. 19 Protons Electrons Answer! One Valence Electron 2 8 8 1
- 261. • What element is this? – Why?
- 262. • What element is this? – Why? – Trick, not a real atom.
- 263. • What element is this? – Why? – Trick, not a real atom. Based on valence electrons it would be nitrogen with 5.
- 264. • What element is this? – Why? – Trick, not a real atom. Based on valence electrons it would be nitrogen with 5. Based on total electrons it should be neon with 10.
- 265. • What element is this? – Why? – Trick, not a real atom. Based on valence electrons it would be nitrogen with 5. Based on total electrons it should be neon with 10. My best guess is that it’s suppose to be neon but the creator in cyberland does not know about electron orbitals.
- 266. • Activity! Online Atom Builder. • http://www.freezeray.com/flashFiles/atomB uilder.htm • http://www.sharewareconnection.com/the- atom-builder.htm – Sodium Na – Calcium Ca – Potassium K – Beryllium Be – Aluminum Al Copyright © 2010 Ryan P. Murphy
- 267. • You should doing page 5 and 6 in your bundle.
- 268. • Additional Available Sheet. Orbitals, – Find P+, N, and E-, Atomic #, Mass and more.
- 271. http://sciencepowerpoint.com/Atoms_Periodic_Table_of_Elements_Unit.html Areas of Focus within The Atoms and Periodic Table Unit: Atoms (Atomic Force Microscopes), Rutherford’s Gold Foil Experiment, Cathode Tube, Atoms, Fundamental Particles, The Nucleus, Isotopes, AMU, Size of Atoms and Particles, Quarks, Recipe of the Universe, Atomic Theory, Atomic Symbols, #’;s, Valence Electrons, Octet Rule, SPONCH Atoms, Molecules, Hydrocarbons (Structure), Alcohols (Structure), Proteins (Structure), Atomic Bonds, Ionic Bonds, Covalent Bonds, Metallic Bonds, , Precipitation Reactions, Acids and Bases, Electron Negativity, Polar Bonds, Chemical Change, Exothermic Reactions, Endothermic Reactions, Laws Conservation of Matter, Balancing Chemical Equations, Oxidation and Reduction, Periodic Table of the Elements, Organization of Periodic Table, Transition Metals, Acids and Bases, Non-Metals, Metals, Metalloids, Ionization.
- 277. • This PowerPoint roadmap is one small part of my Atoms and Periodic Table Unit. • This unit includes a four part 2000+ slide PowerPoint roadmap. • 13 page bundled homework that chronologically follows slideshow • 14 pages of unit notes with visuals. • 3 PowerPoint review games. • Activity sheets, rubrics, advice page, curriculum guide, materials list, and much more. • http://sciencepowerpoint.com
- 279. • Please visit the links below to learn more about each of the units in this curriculum – These units take me about four years to complete with my students in grades 5-10. Earth Science Units Extended Tour Link and Curriculum Guide Geology Topics Unit http://sciencepowerpoint.com/Geology_Unit.html Astronomy Topics Unit http://sciencepowerpoint.com/Astronomy_Unit.html Weather and Climate Unit http://sciencepowerpoint.com/Weather_Climate_Unit.html Soil Science, Weathering, More http://sciencepowerpoint.com/Soil_and_Glaciers_Unit.html Water Unit http://sciencepowerpoint.com/Water_Molecule_Unit.html Rivers Unit http://sciencepowerpoint.com/River_and_Water_Quality_Unit.html = Easier = More Difficult = Most Difficult 5th – 7th grade 6th – 8th grade 8th – 10th grade
- 280. Physical Science Units Extended Tour Link and Curriculum Guide Science Skills Unit http://sciencepowerpoint.com/Science_Introduction_Lab_Safety_Metric_Methods. html Motion and Machines Unit http://sciencepowerpoint.com/Newtons_Laws_Motion_Machines_Unit.html Matter, Energy, Envs. Unit http://sciencepowerpoint.com/Energy_Topics_Unit.html Atoms and Periodic Table Unit http://sciencepowerpoint.com/Atoms_Periodic_Table_of_Elements_Unit.html Life Science Units Extended Tour Link and Curriculum Guide Human Body / Health Topics http://sciencepowerpoint.com/Human_Body_Systems_and_Health_Topics_Unit.html DNA and Genetics Unit http://sciencepowerpoint.com/DNA_Genetics_Unit.html Cell Biology Unit http://sciencepowerpoint.com/Cellular_Biology_Unit.html Infectious Diseases Unit http://sciencepowerpoint.com/Infectious_Diseases_Unit.html Taxonomy and Classification Unit http://sciencepowerpoint.com/Taxonomy_Classification_Unit.html Evolution / Natural Selection Unit http://sciencepowerpoint.com/Evolution_Natural_Selection_Unit.html Botany Topics Unit http://sciencepowerpoint.com/Plant_Botany_Unit.html Ecology Feeding Levels Unit http://sciencepowerpoint.com/Ecology_Feeding_Levels_Unit.htm Ecology Interactions Unit http://sciencepowerpoint.com/Ecology_Interactions_Unit.html Ecology Abiotic Factors Unit http://sciencepowerpoint.com/Ecology_Abiotic_Factors_Unit.html
- 282. • The entire four year curriculum can be found at... http://sciencepowerpoint.com/ Please feel free to contact me with any questions you may have. Thank you for your interest in this curriculum. Sincerely, Ryan Murphy M.Ed www.sciencepowerpoint@gmail.com
