E e experiments
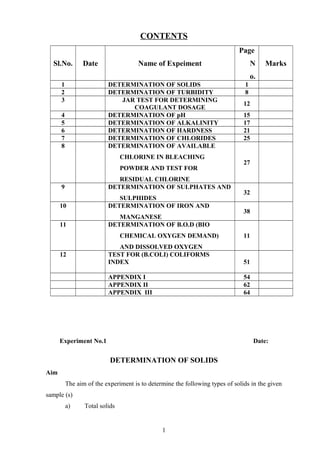
- 1. CONTENTS Sl.No. Date Name of Expeiment Page N o. Marks 1 DETERMINATION OF SOLIDS 1 2 DETERMINATION OF TURBIDITY 8 3 JAR TEST FOR DETERMINING COAGULANT DOSAGE 12 4 DETERMINATION OF pH 15 5 DETERMINATION OF ALKALINITY 17 6 DETERMINATION OF HARDNESS 21 7 DETERMINATION OF CHLORIDES 25 8 DETERMINATION OF AVAILABLE CHLORINE IN BLEACHING POWDER AND TEST FOR RESIDUAL CHLORINE 27 9 DETERMINATION OF SULPHATES AND SULPHIDES 32 10 DETERMINATION OF IRON AND MANGANESE 38 11 DETERMINATION OF B.O.D (BIO CHEMICAL OXYGEN DEMAND) AND DISSOLVED OXYGEN 11 12 TEST FOR (B.COLI) COLIFORMS INDEX 51 APPENDIX I 54 APPENDIX II 62 APPENDIX III 64 Experiment No.1 Date: DETERMINATION OF SOLIDS Aim The aim of the experiment is to determine the following types of solids in the given sample (s) a) Total solids 1
- 2. b) Total (inorganic) fixed solids c) Total volatile (organic) solids d) Total dissolved solids e) Dissolved fixed (inorganic) solids f) Dissolved volatile (organic) solids g) Total suspended solids h) Suspended fixed (inorganic) solids i) Suspended volatile (organic) solids j) Settleable solids Principle Total solid is the term applied to the material left in the vessel after evaporation of sample of water/waste water and its subsequent drying in an oven at a definite temperature. Total solids include “ total suspended solids” the portion of the total solids retained by a filter and total “dissolved solids” the portion that passes through the filter. Fixed solid is the residue remaining after ignition for 1 hour at 5500 C. The solid portion that is volatilized during ignition is called Volatile solids. It will be mostly organic matter Water that are low in organic matter and total mineral content and are intended for human consumption may be examined under 103-1050 or 179-1810 C. but waters containing considerable organic matter or those with pH over 9.0 should be dried at 179- 1810 C. in any case the report should indicate the drying temperature. The sample is evaporated in weighed dish on a steam bath and then is dried to constant weight in an oven at either 103-1050 C or 179-1810 C. the increase in weight over that of the empty dish represents the total solids. The sample is filtered and the filtrate evaporated in a weighted dish on steam path. The residue left after evaporation is dried to constant weight in an oven at either 103-1050 or 179-1810 C. the increase in weight over that of the empty dish represents the total dissolved solids and includes all materials, liquid or solid, in solution or otherwise, which pass through the filter and not volatilized during the drying process. The difference between the total solids and the total dissolved solids will give the total suspended solids. 2
- 3. The dishes with the residue retained after completion of the test for total solids and total dissolved solids are subjected to heat for 1 hour in a muffle furnace held at 5500 C. the increase in weight over that of the ignited empty vessel represents fixed solids in each instance. The difference between the total dissolved/total suspended solids and the corresponding fixed solids will give the volatile solids in each instance. All the quantity should be expressed in mg./L Setteleable matter in surface and saline waters as well as domestic and industrial waters may be determined and reported on a volume basis milliliters per liter. Apparatus 1. Porcelain evaporating dishes of 150-200ML capacity 2. Steam bath 3. Drying oven 4. Desiccator 5. Analytical balance or monopan balance 6. Filter paper (preferable of glass fiber) 7. Electric muffle furnace 8. Imhoff cons Procedure a) Total Solids 1. Ignite the clean evaporating dishes in the muffle furnace for 30 minutes at 5500 C and cool in desiccator. 2. Note down the empty weight of the dish (W1) 3. Pour a measured portion (50 or 100ML) of the well-mixed sample into the dish and evaporate the contents by placing the dish on a stem bath. 4. Transfer the dish to an oven maintained at either 103–050 C or 179-810 C and dry it overnight. 3
- 4. 5. Allow the dish to cool briefly in air before placing it, while still warm, in a desiccator to complete cooling in a dry atmosphere. 6. Weigh the dish as soon as it has completely cooled (W2) 7. Weight of residue = (W2-W1) mg. W2 and W1 should be expressed in mg. b) Total Fixed Solid 1. Keep the same dish used for determining total residue in a muffle furnace for I hour at 5500 C. 2. Allow the dish to partially cool in air until most of the heat has disappeared, then transfer to a desiccator for final cooling in a dry atmosphere. 3. Weigh the dish as soon as it has cooled (W3) 4. Weight of the total fixed residue = (W3-W1) mg W3 W1 should be expressed in mg. 4
- 5. c) Total Dissolved Solids 1. Filter a measured portion of the mixed sample (50 or 100ML) through a filter paper and collect the filtrate in a previously prepared and weighed evaporation dish. 2. Repeat the steps 3 to 6 outlined to total solid procedure. 3. Weight of dissolved solids – (W5-W4) mg. W4= weight of empty evaporating dish in mg. W=Weight of empty evaporating dish in mg + residue left after evaporating the filtrate in mg. d) Total Suspended Solids = Total solids – Total dissolved solids e) Total Volatile Solids = Total solids – Total fixed solids. f) Fixed Dissolved Solids 1. Keep the same evaporating dish used in determining total dissolved solids in a muffle furnace for 1 hour at 5500 C. 2. Repeat the steps 2 and 3 outlined in total fixed solids procedure. 3. Weight of fixed dissolved solids = (W6 -W4) mg. W6 = weight empty evaporating dish + fixed solids left after ignition at 5500 C. g) Volatile Dissolved Solids Total dissolved solids – fixed dissolved solids h) Fixed Suspended Solids Total fixed solids –Fixed dissolved solids i) Volatile Suspended Solids Total volatile solids – volatile dissolved solids 5
- 6. j) Settleable solids: by volume 1. Fill an imhoff cone to the liter mark with a thoroughly mixed sample 2. Settle for 45 minutes 3. Gently stir the solids of he cone with a rod or by spinning. 4. Settle 15 minutes longer. 5. Record the volume of settleable matter in the cone as mL/L Observations No. Item Sample No. or Description 1 Volume of sample taken 2 Wt. of empty evaporating dish = W1 mg (for total solids) 3 Wt. of dish + total solids = W2mg 4 Total solids = (W2 –W1) mg 5 Wt. of dish + fixed solids = W3 in mg 6 Fixed solids in mg = (W3-W1) 7 Wt. of empty evaporating dish = W4 mg ( for total dissolved solids) 8 Wt. of dish + total dissolved solids = W5 mg 9 Total dissolved solids = (W5 – W4) mg 10 Wt. dish + fixed dissolved solids = W6 mg 11 Fixed dissolved solids = (W6 –W4) mg 12 Total solids in mg./ L 13 Total fixed solids in mg./ L 14 Total dissolved solids in mg./ L 15 Total suspended solids in mg./ L 16 Total volatile solids in mg./ L 17 Fixed dissolved solids in mg./ L 18 Volatile dissolved solids in mg./ L 19 Fixed suspended solids in mg./ L 20 Volatile suspended solids in mg./ L 21 Settleable solids in mg./ L 6
- 7. Calculation 1 Mg/L total solids = Mg total solids x 1000 = ML of sample = 2 Mg/L total fixed solids = Mg. Total fixed solids x 1000 = ML of sample = 3 Mg/L total dissolved solids = Mg of total dissolved solids x 1000 = Ml of sample = 4 Mg/L total suspended solids = Mg/L of total solids - Mg/L of total dissolved solids = 5 Mg/L total volatile solids = Mg/L of total solids - Mg/L of total fixed solids = 6 Mg/L fixed dissolved solids = Mg fixed dissolved solids x 1000 = ML of sample = 7 Mg/L volatile dissolved solids = Mg/L total dissolved solids - Mg/L of fixed dissolved solids = 8 Mg/L fixed suspended solids = Mg/L total fixed solids - Mg/L fixed dissolved solids = 9 Mg/L volatile suspended solids = Mg/L total volatile solids - m/L volatile dissolved solids Note: - This calculation need be shown only for one sample 7
- 8. Results No Item Sample No. or Description 1 Mg/L of total solids 2 Mg/L of total fixed solids 3 Mg/L of total dissolved solids 4 Mg/L of total suspended solids 5 Mg/L of total volatile solids 6 Mg/L of fixed dissolved solids 7 Mg/L of volatile dissolved solids 8 Mg/L fixed suspended solids 9 Mg/L volatile suspended solids 10 ML/ L of settleable solids Discussion 8
- 9. Experiment No. 2 Date: DETERMINATION OF TURBIDITY Aim The aim of the experiment is to determine the turbidity of given sample (s) by using Jackson Candle turbidity meter and photoelectric turbidity meter. Principle Turbidity in water is caused by the presents of suspended matter, such as clay, silt, finely divided organic and inorganic matter, plankton and other microscopic organisms. Turbidity should be clearly under stood to be an expression of the optical property of a sample which causes light to be scattered and absorbed rather than transmitted in straight line through the sample. Attempts to correlate the turbidity with the weight concentration of suspended matter are impractical as the size, shape and refractive index of the particulate materials are of grate importance optically but bear little direct relationship to the concentration and specific gravity of the suspended matter. The standard method for the determination of turbidity has been based on the Jackson Candle turbidity meter. However the lowest turbidity value, which can be measured on this instrument, is 25 units. With treated water generally falling within the range of 0.5 units, indirect secondary methods have been required to estimate turbidities on such samples. Photoelectric turbidity meter is one such instrument. Turbidity measurements by the candle turbidity meter are based on the light path through a suspension, which just causes the image of the flame of a standard candle to disappear, that is, to become in distinguishable against the general background illumination when the flame is viewed through the suspension. The longer the light path, the lower the turbidity. Measurements of turbidity using the photoelectric turbidity meter is based upon a comparison of the intensity of light scattered by the sample under defined condition with the intensity of light scattered by a standard reference suspension under same conditions. 9
- 10. Apparatus 1. Jackson candle turbid meter consisting of a calibrated glass tube, a standard candle and a support, which aligns the candle, and the tube. 2. Photoelectric turbidity meter. Reagents 1. Turbidity free distilled water Procedure a) Using Jackson candle turbidity meter 1. Pour the shaken sample in to the glass tube. 2. Light the candle and observe the image of the candle flame. 3. If the flame is seen through the solution, pour some more shaken sample into the tube till the image of the candle flame disappears. At this stage make certain that a uniformly illuminated field with no bright spot materializes. 4. Remove 1 percent of the sample to make the flame image visible again. 5. Employ a pipit to add the small amounts of the sample till the flame image disappears. 6. Note down the reading in the glass tube as the turbidity of the given sample in Turbidity Units. 7. Prepare 2 or 3 dilutions of the sample and find out the turbidity as outlined in steps 1 to 6. Observation (For Jackson candle turbid meter) Sample No. or Description Height of end point in cm. Turbidity units 10
- 11. b) Using Photoelectric Turbidity Meter 1. Follow the manufacture’s operating instructions. 2. In the absents of a pre-calibrated graph, prepare calibration curves. 3. Immediately after finding the turbidity of a sample in the Jackson Candle turbidity meter, keep same sample in the photoelectric turbidity meter and note down the instrument reading. 4. Find out the instrument reading for 2 to 3 dilutions of the same sample for the corresponding turbidity units. 5. Prepare a graph of turbidity units Vs instrument readings. 6. Use only the straight-line portions of the graph for future use. 7. Measure the turbidity of the other sample taken from the same source using the instrument and the calibration graph. Reading for Calibration Graph Sample No. or Description Turbidity Meter Reading 11
- 12. Result Sample No. or Description Turbidity Units Discussion 12
- 13. Experiment No.3 Date: JAR TEST FOR DETERMINING COAGULANT DOSAGE Aim The aim of the experiment is to determine the optimum coagulant dose for clarifying the given sample of water by using alum as the coagulant and perform the jar test. Principle Coagulants are used in water treatment plants to i) Remove natural suspended and colloidal matters ii) Remove materials which do not settle in plain sedimentation and iii) To assist in filtration Alum is the mostly widely used coagulant. Now a days coagulant aids are also being used in conjunction with alum to reduce the alum does required. Jar test is a simple device, which will help in determining the optimum coagulant dose required. It has got its own limitations in spite of the best efforts to simulate treatment plant conditions in the jar test. It still remain all odds the most valuable economical dosage for given water. By experience, a plant factor can be found out and after applying to the coagulant dose obtained by the jar test, the actual dose required for the treatment plant can be determined. This test is supposed to be conducted as a routine experiment in all the water treatment plants. The jar test, device consists of a number of stirrers (4 to 6) provide with paddles. The paddles can be rotated with varying speed with the help of a motor and a regulator. Samples will be taken in jars or beakers and varying doses of coagulant will be added simultaneously to all jars. The paddles will be rotated at 100 rpm for 1 minute and at 40 rpm for 9 minutes, corresponding to the flash mixing and slow mixing in the flocculator of the treatment plant. After 10 minutes setting, supernatant will carefully decant from all the jars, to measure the turbidity. The dose, which gives the least turbidity, will be taken as the optimum coagulant dose. 13
- 14. Apparatus 1. Jar testing apparatus (laboratory flocculator) 2. Turbidity meter 3. Beakers, pipette, etc. Reagents 1. 1 percent alum solution Procedure 1. Measure initial turbidity using J.C.T meter 2. Measure 500mL of the sample in the 4 beakers and place them in the jar testing apparatus. 3. Switch on the instrument and adjust the speed of the paddles to 100 rpm. 4. Measure and adjust the pH of the sample between 6 & 8 5. Add varying doses of alum in the increasing order corresponding to 1.2, 4, 8 mg/L to the beakers, simultaneously and start a stopwatch. 6. Allow the rapid mix at 100 rpm for 1 minute. 7. Bring down the speed to 40 rpm. And allow the slow mix for 9 minutes. 8. Switch off the instrument and allow 10 minutes setting. 9. Take out the supernatant (50mL) with out disturbing the settle flocs. If possible simultaneously from all the beakers. 10. Measure the turbidity of all the samples with the help of a turbidity meter. 11. Repeat the steps 1 to 8 with higher doses of alum if necessary. 12. Draw a graph of settled water turbidity with alum dose. 13. Note down the economical or optimal dose from the graph. 14
- 15. Sl. No. Sample No. or Description Volume of sample taken Dose of Coagulant added Settled Water Turbidity Result The optimum alum dose for the given sample of water = __________mg/L Discussion 15
- 16. Experiment No.4 Date: DETERMINATION OF PH Aim The aim of the experiment is to determine the pH given sample(s) using pH meter and also pH paper. Principle pH is the logarithm of the reciprocal of the hydrogen ions concentration more precisely of the hydrogen ion activity in moles/liter. pH enters into the calculation of carbonate. Bicarbonate and carbon – dioxide, as well as of the corrosion or stability index and into the control of water treatment process. The practical pH scale extends from 0, very acidic to 14, vary alkaline, with the middle value (pH7) corresponding to exact neutrality at 250 C. where as alkalinity and acidity express, the total reserve or buffering capacity of sample, the pH value represents the instantaneous hydrogen ion activity i.e. the intensity of acidity or alkalinity. The pH meter makes use of electrodes for measuring pH of sample. Several types of electrodes have been suggested for electrometric determination of pH. Although the hydrogen gas electrode is recognized as the primary standard, the glass electrode in combination with the reference potent ional provided with saturated calomel electrode is most generally used. The glass electrode system is based on the fact that a change of 1 pH unit produces an electrical change of 59.1mv at 250 C. The pH paper is a specially prepared one, which will show the variation in pH with different colour changes. The method is suitable for only rough estimation. Apparatus 1. pH meter with electrodes 2. Buffer solutions 3. Thermometer 4. pH papers or pH colour comparator 16
- 17. Procedure a) Using a pH Meter 1. Follow the manufactures operating instructions. 2. Calibrate the instrument with a buffer solution. (buffer solution is one whose pH is already known and which will retain that pH for a long time). 3. Dip the electrode in the given sample and note down the instrument reading. 4. Note the pH of the sample along with its temperature. b) Using pH paper 1. Dip the pH paper in the sample. 2. Compare the colour with that of the colour given on the wrapper of the pH paper book. 3. Note down the pH of the sample along with its temperature. c) Using colour comparator 1. Fill the left glass with distilled water or sample up to 10ml marl 2. Fill the right glass tube with the sample up to 10 ml mark. 3. Note the temperature of the sample. 4. Add 4 drops of pH universal indicator into the right glass tube and mix it well. 5. Make the colour of the sample with that of the disk. 6. Note the pH value shown in the lower window of the colour comparator. Result Sample No. or Description Temp. in 0 C PH using pH meter PH using pH paper or colour comparator Discussion 17
- 18. Experiment No.5 Date: DETERMINATION OF ALKALINITY Aim The aim of the experiment is to determine which of the following types of alkalinity are present in the given sample a) Hydroxide alkalinity b) Carbonate alkalinity c) Bicarbonate alkalinity d) Hydroxide - carbonate alkalinity e) Carbonate – Bicarbonate alkalinity Principle The alkalinity of water is the capacity of water to accept protons. Alkalinity is usually imparted by the bicarbonate, carbonate and hydroxide components of natural or treated water supply. It is determined by titration with a standard solution of strong mineral acid to the successive bicarbonate and carbonic acid equivalence points, indicated electrometrically or by means of colour. Phenolphthalein indicator enables the measurement of the alkalinity fraction contributed by the hydroxide and half of the carbonate. Methyl orange indicator will help in measuring the remaining carbonate and bicarbonate fractions of alkalinity Alkalinity is expressed in mg/L CaCO3. Apparatus 1. Burette 25 to 0mL capacity 2. Erlenmeyer flasks 3. Pipettes 18
- 19. Reagent a) Carbon dioxide free distilled water b) Phenolphthalein indicator solution (i) c) 0.02N standard sulphuric acid (iii) d) Methyl orange indicator solution (ii) e) 0.1N sodium thiosulphate solution (iv) Procedure 1. Measure out 50mL of the given sample to an Erlenmeyer flask 2. Add 1 drop of 0.1N sodium thiosulphate solution to remove the free residual chlorine if present. 3. Add two drops of phenolphthalein indicator. 4. If the sample turns pink, then titrate with 0.02N standard sulphuric acid till the solution turns colourless. 5. Note down the volume of sulphuric acid added (V1) 6. Add 2 drops of methyl orange indicator to the solution in which the phenolphthalein alkalinity has been determined. 7. If the solution turns yellow, continue titration with 0.02N standard sulphuric acid till the solution turns faint orange in colour. 8. Note down the total volume of sulphuric acid added (V2) Calculation 1. Phenolphthalein Alkalinity (P) as mg./L CaCO3 = V1 x N x 50,000 mL of sample = 2. Total Alkalinity ( T ) as mg/L CaCO3 = V2 x N x 50,000 mL of sample The type of alkalinity present in the samples is calculated using the equations given in the Table I and the result are tabulated. 19
- 20. Result of Titration Hydroxide alkalinity as CaCO3 Carbonate alkalinity as CaCO3 Bicarbonate alkalinity as CaCO3 P = 0 0 0 T P< ½ T 0 2P T – 2P P = ½ T 0 2P 0 P > ½ T 2P – T 2 (T-P) 0 P = T T 0 0 20
- 21. Observations Sample No. or Description Volume of acid added = V1 in mL Total volume of acid added = V2 in mL P –Alkalinity in mg/L as CaCO3 T – Alkalinity in mg/L as CaCO3 Results Type of Alkalinity Sample No. or Description Hydroxide Alkalinity in mg/L as CaCO3 Carbonate Alkalinity in mg/L as CaCO3 Bicarbonate Alkalinity in mg/L as CaCO3 Discussion 21
- 22. Experiment No.6 Date: DETERMINATION OF HARDNESS Aim The aim of the experiment is to determine the total hardness of the given sample(s) by (i) Using soap solution (ii) EDTA Titrimerric Method Principle Originally the hardness of water was understood to be a measure the capacity of the water for precipitating soap. Soap is precipitated chiefly by the calcium and Magnesium ions, commonly present in water, but may also be precipitated by ions of other polyvalent metals, such as aluminum, iron, manganese, strontium and zinc and by hydrogen ions. Because all but the first two are usually present in insignificant concentrations in natural waters, hardness is defined as a characteristic of water which represents the total concentration of just the calcium and magnesium ions expressed as calcium carbonate. However, if present in significant, other hardness producing metallic ions should be included. When the hardness is numerically grater than the sum of the carbonate alkalinity and the bicarbonate alkalinity, the amount of hardness which is equivalent to the total alkalinity is called Carbonate Hardness, the amount of hardness is excess of this is called Non- Carbonate Hardness. When the hardness is numerically equal to or less than the sum of carbonate and bicarbonate alkalinity all of the hardness is carbonate hardness and there is no non-carbonate hardness. The hardness may range from Zero to Hundreds of milligrams per liter in terms of calcium carbonate, depending on the source and treatment to which the water has been subjected. Ethylene diamine tetra acetic acid and its sodium salts (EDTA) from a chelated soluble complex when added to a solution of certain meta cations. If a small amount of dye such as Eriochrome black T is added to an aqueous solution containing calcium and magnesium ions at a pH of 10 + 0.1, the solution will become wine red. If EDTA is then added as a titrant, the calcium and magnesium will be complexed. After sufficient EDTA has been to added to complex all the magnesium and calcium, the solution will turn from wine red to blue. This is the end point of the titration. 22
- 23. Apparatus 1. Burette 2. Pipette 3. Erlenmeyer flask 4. Bottle etc. Reagents 1. Standard soap solution (xx) 2. Ammonia buffer solution (V) 3. Eriochrome black T indicator (vi) 4. Standard EDTA titrant (0.01 M) (vii) i). Determination to Hardness by soap solution method. Procedure 1. Pipette 50ml of the sample in to a bottle 2. Add the standard soap in small portions, shaking vigorously after each addition 3. As the end point is approached, the soap solution should be added drop by drop 4. After permanent lather is produced which will last for 5 minutes. With the bottle on its side, stop the titration. 5. Record the mL of soap solution used. 6. Continue the addition of small quantities of soap solutions. If the lather is again disappears, first end point was false owing to the presence of magnesium salts. 7. Continue the addition of the soap solution until the true end point is reached and record the mL of soap solution used. 8. Take 50mL of distilled water in a bottle. 9. Titrate it against standard soap solution till the end point is reached. 10. Note down the volume of solution added as the lather factor 23
- 24. Observations Sample No or Description Volume of sample taken Volume of soap solution consumed for the sample Volume of soap solution consumed for distilled water Volume of EDTA consumed Calculation Total Hardness in = (ml. of soap solution for the sample – lather factor) x 1000 mg/L CaCO3 mL of sample ii) Determination of hardness EDTA Titrimetric Method. Procedure 1. Dilute 25mL of sample to about 50mL with distilled water in an Erlenmeyer flask. 2. Add 1ML of buffer solution 3. Add 2 drops of indicator solution. The solution turns wine red in colour 4. Add the standard EDTA Titrant slowly, with continuous stirring, until the last reddish tinge disappears from the solution. The colour of the solution at the end point is blue under normal conditions. 5. Note down the volume of EDTA added (A) Calculation Hardness (EDTA) as mg/L CaCO3 A X B X 1000 ml. of sample Note: A = ml of EDTA consumed B = mg CaCO3 equivalent to 1ml EDTA titrant = 1mg. CaCO3 24
- 25. Result Sample No. or Description Total Hardness in mg/L as CaCO3 By Soap Solution Method By EDTA Method Discussion 25
- 26. Experiment No.7 Date: DETERMINATION OF CHLORIDES Aim The aim of the experiment is to determine the amount of chlorides present in the given sample (s) by Argentomeric method Principle In nature or slightly alkaline solution, potassium chromate can indicate the end point of the silver nitrate titration of chloride. Silver chloride is quantitatively precipitated before red silver chromate is formed. Apparatus 1. Burette 2. Pipette 3. Erlenmeyer flask Reagents 1. Chloride – free distilled water 2. Potassium chromate indicator (xiii) 3. Standard silver nitrate titrant (0.0141 N) (xiv) 4. Standard sodium chloride (0.0141 N) ( xv) Procedure 1. Take 100 mL sample in an Erlenmeyer flask. 2. If the sample is highly coloured, add 3mL [AL (OH)3], suspension mix, allow to settle, filter, wash and combine filtrate and washing. 3. Titrate samples in the pH range 7-10 directly. Adjust the samples not in this range with sulphuric acid or sodium hydroxide solution. 4. Add 1 mL potassium chromate indicator solution. 5. Titrate with standard silver nitrate titrant to a pinkish yellow end point. 6. Note down the volume of silver nitrate titrant added (A) 7. Take 100 mL distilled water in another Erlenmeyer flask and repeat the procedure outlined in step 3 to 5 above. 8. Note down the volume of silver nitrate titrant added (B) 26
- 27. Calculation Mg./L chloride = (A-B) x N x 35450 ML of sample taken N = Normality of silver nitrate titrant. = 0.0141 Observations Sample No. Or Description Volume of sample taken A = Volume of silver nitrate titrant added B = Volume of silver nitrate added for blank correction Results Sample No. Or Description Chlorides present in mg/L Discussion 27
- 28. Experiment No. 8 Date: DETERMINATION OF AVAILABLE CHLORINE IN BLEACHING POWDER AND TEST FOR RESIDUAL CHLORINE Aim The aim of the experiment is to determine the available chlorine in the given sample of Bleaching Powder by using iodometric method and to find the chlorine dosage for the given sample of water by using ortotolodine method in a chloroscope. Principle Bleaching Powder is commonly used as a disinfectant in many small water treatment plants. To find the exact dose of Bleaching Powder it essential to find out the amount of available chlorine in the Bleaching Powder sample. As the chlorine present in Bleaching Powder gets reduced with time, this test should always be conducted before adding Bleaching Powder to water. The iodometric method is considered the standard against which other methods are judged. It provides the means for standardizing the chlorine water used in preparing temporary standards. Chlorine will liberate free iodine from potassium iodide solutions when its pH is 8 or less. The liberated iodine is titrated with a standard solution of sodium thiosulphate, using starch as indicator. The reaction is preferably carried out at pH 3 to 4 The ortotolodine method measures both free and combined available chlorine. To obtain the correct colour development with chlorine and ortotolodine, (a) the solution must be at pH 1.3 or lower during the contact period (b) the ratio by weight of ortotolodine to chlorine must be at least 3:1 and (c) the chlorine concentration must not exceed 10mg./L. the ortotolodine reacts with chlorine residual and gives yellow colour to the sample. The intensity of colour formation is proportional to the amount of chlorine residual present. Usually permanent colour standards representing different values of chlorine residuals will be prepared and kept ready for comparison. 28
- 29. Apparatus 1. Burette 2. Pipette 3. Erlenmeyer flask 4. Chloroscope etc. Reagents 1. Acetic acid, concn (glacial) 2. Potassium iodide crystals 3. Standard sodium thiosulphate solution (0.025N) (xii) 4. Ortotolodine reagent (xvi) 5. Starch indicator (x) Procedure a) Available chlorine in Bleaching Powder using iodometric method 1. Dissolve 1g. Bleaching Powder in 1 liter of distilled water and stopper the container 2. Place 5 ml acetic acid in an Erlenmeyer flask and add about 1g. potassium iodide crystals. Pure in 25ml of Bleaching Powder solution prepared above and mix with the stirring rod. 3. Titrate with 0.025N sodium thiosulphate solution until the yellow colour of the liberated iodine is almost discharged. 4. Add 1 mL starch solution and titrate until the blue colour disappears. 5. Note down the volume of sodium thiosulphate solution added (A). 6. Take a volume of distilled water corresponding to the sample used. 7. Add 5 mL acetic acid, 1g. Potassium iodide and 1 mL starch solution. 8. If the blue colour occurs, titrate with 0.025N sodium thiosulphate solution till the blue colour disappears. 9. Record the volume of sodium thiosulphate solution added. 10. If no blue colour occurs, titrate with 0.025N iodine solution until a blue colour appears. Then titrate with 0.025N sodium thiosulphate solution till the blue colour disappears. 11. Record the volume of sodium thiosulphate solution added. Note down the different between the vol.of iodine solution and sodium thiosulphate as (B2) 29
- 30. Note : Blank titration is necessary to take care of i) The oxidizing or reducing reagent impurities ii) The iodine bound to starch at the end point. Calculations Mg/ml Cl = [(A-B1) or (A+B2)] x N x 35.45 = Ml of Bleaching Powder solution taken N = Normality of sodium thiosulphate solution = 0.025 000 mL of Bleaching Powder solution contains = 1000 x ______ mg. of chlorine = i.e. 1000mg of Bleaching Powder contains ______ mg of chlorine 100 mg of Bleaching Powder contains _______ mg of chlorine. Percentage of chlorine available in Bleaching Powder = _______ (x) 30
- 31. B) Determination of Chlorine Dosage for the Given Water Sample Using the above Bleaching Powder Solution. 1. Measure 200mL of the given sample of water into 5 Erlenmeyer flasks of ample accuracy. 2. Add suitable increasing amounts of Bleaching Powder solution to the successive Erlenmeyer flask in such a way that the chlorine add will be 0.1mg/L, 0.2mg/L, 0.5mg/l, 1mg/L and 1.5mg/L as mentioned in step 4 below. 3. Mix while the chlorine solution is being added to the sample. 4. Dose the portions of the sample according to a staggered schedule that will permit the determination of chlorine residuals at 30 minutes contact time. 5. At the end of contact period, pour the solution in the flask into the middle chamber of the cell of the chloroscope. Add 5 drops of orthotolodine solution and mix well with the plunger. Fill the outer chambers with unchlorinated sample of water, leave for 5 minutes. 6. Put the glass discs which indicate the does of chlorine on its top in front of the outer chambers. 7. Compare the colour of the middle chamber with the colour of the glass discs. 8. The disc which matches with the colour of the sample will give the chlorine residual present in the sample. Calculation Select the lowest dose which gives 0.2mg/L of residual chlorine as the chlorne dosage (y) = __________ Chlorine demand = Amount of chlorine added – 0.2mg/L residual chlorine Amount of Bleaching Powder required per liter of water sample = 100 x (y) = ___________ X 31
- 32. Observation Chlorine added in mg/L Residual chlorine in mg/L Results 1. Available chlorine in the given Bleaching Powder = 2. Chlorine demand of the water sample = 3. Chlorine dosage of the water sample = 4. Bleaching Powder required to treat 1 liter of water sample = Discussions 32
- 33. Experiment No. 9 Date: DETERMINATION OF SULPHATES AND SULPHIDES A) DETERMINATION OF SUPLPHATES Aim The aim of the experiment is to determine the amount of sulphates presents in the given sample (s) by Gravimetric with ignition of residue. Principle Sulphate is precipitated in a hydrochloric acid medium as barium sulphates by the addition of barium chloride. The precipitation is carried out near the boiling temperature and after a period of digestion the precipitates is filtered, washed with water until free of chloride, ignited and weighed as barium sulphates. Apparatus 1. Drying oven 2. Desiccator 3. Steam bath 4. Analytical balance 5. Ash less filter paper (What man filter paper No. 42) 6. Muffle Furnace 7. Glass ware like funnel, flask, and pipet Reagents 1. Methyl Red indicator solution ( xxxv) 2. Hydrochloric acid, HCL 1+1 3. Barium chloride solution ( xxxvi) 4. Silver nitrate nitric acid reagent (xxxvii) 33
- 34. Procedure 1. Take 250 ml of the sample in a conical flask 2. Adjust the acidity with HCL to pH 4, 5 to 5 using a pH meter or the orange colour of methyl red indicator. 3. Then add an additional 1 to 2 mL HCL. 4. Heat the solution to boiling and while stirring gently add barium chloride solution. Slowly until precipitation appears to be complete. Then add about 2 mL in excess. 5. Digest the precipitate at 30 to 900 C preferable overnight but for not less than two hours. 6. Filter the contents in the flask through an ash less filter paper. 7. Wash the precipitate with small portions of warm distilled water until the washings are free of chloride as indicated by testing with silver nitrate- nitric acid reagent. 8. Place the precipitate along with the filter paper in a crucible after finding its empty weight and dry it. 9. Keep the crucible in a muffle furnace and ignite at 8000 C for 1 hour. 10. Cool in a desiccator and weigh. 11. Find the weight of the barium precipitate. Calculation Mg/L SO4 = mg. BaSO4 x 411.5 Ml of sample = 34
- 35. Observations Sample No or Description Volume of sample Empty weight of the crucible Wt. of crucible + residue after ignition Wt. of BaSO4 precipitate Mg/L SO4 Result Sample No or Description Mg/L of SO4 Discussion 35
- 36. B) DETERMINATION OF SULPHIDES Aim The aim of the experiment is to determine the amount of sulphides present in the sample(s) by T itrimetric (Iodine) method. Principle Sulphide is often present in ground water, especially in hot springs an is common in waste waters, coming in part from the decomposition of organic matter, some times from industrial wastes, but mostly from the bacterial reduction of sulphate. Hydrogen sulphide escaping into the air from sulphide containing wastewater causes odour nuisances. The threshold odour concentration of hydrogen sulphides in clean water is between 0.01 and 0.1/ug/L. H2S is very toxic and has claimed the lives of numerous workmen in sewers. It attacks metals directly and indirectly has caused serious corrosion of concrete sewers because it is oxidized biologically to sulphuric acid on the pipe wall. Iodine reacts with sulphide in acid solution, oxidizing it to sulpher. A titration based on this reaction is an accurate method for determining sulphide at concentrations above 1mg/L if interferences are absent and if loss H2S is avoided. Apparatus 1. Burette 2. Pipette 3. Conical Flasks etc. Reagents 1. Hydrochloric acid, HCl, 6N 2. Standard iodine solution (0.025 N) (xxxviii) 3. Standard sodium thiosulphate solution (0.025N) ( xi) 4. Starch solution (x) 36
- 37. Procedure 1. Measure from a burette 10 ml of iodine into a 500ml flask 2. Add distilled water and bring the volume to 20 ml. 3. Add 2 ml 6N HCL 4. Pipette 200 mL of the sample in to the flask, discharging the sample under the surface of the solution. 5. If the iodine colour disappears, add more iodine so that the colour remains 6. Titrate Sodium thiosulphate solution adding a few drops of starch solution as the end point is approached and continuing until the blue colour disappears. Calculation Mg/L sulphide = 400 (a-b) Ml sample a = ml 0.025 N Iodine used b = ml 0.025N thiosulphate Observations Sample No. or description Vol. of Iodine solution used = (a) Vol. of sodium thiosulphate solution used = (b) Vol. of sample used Mg/Ls 37
- 38. Result Sample No. or description Mg/L (sulphide) in the sample Discussion 38
- 39. Experiment No. 10 Date: DETERMINATION OF IRON AND MANGANESE Aim The aim of the experiment is to determine the quantity of iron present in the given sample(s) by phenanthroline method. Principle Iron may be in true solution in a colloidal state that may be peptised by organic matter, in the inorganic or organic iron complexes, or in a relatively coarse suspended particles. It may be either ferrous of ferric, suspended or filterable. In the phenontharoline method, iron is brought in solution, reduced to the ferrous state by boiling with acid and hydroxylamine, and treated with1, 10 phenanthroline at pH 3.2 to 3.3. Three molecules of phenanthroline chilate each atom of ferrous iron to form an orange red complex. The coloured solution obeys Beers law. Its intensity independent of pH from 3 to 9. A pH between 2.9 and 3.5 ensures rapid colour development in the presents of an access of phenanthroline. Colour standards are stables for at least 6 months. Apparatus 1. Colorimetric equipment : one of the following is required. a) Spectrophotometer, for use at 510nm. Providing a light path of 1 cm or longer. b) Nessler tubes, matched, 100mL, tall form. 2. Glassware like conical flasks pipettes and glass beads 39
- 40. Reagents 1. Hydrochloric acid (xxvi) 2. Hydroxylamine solution (xxvii) 3. Ammonium acetate buffer solution (xxviii) 4. Sodium acetate solution (xxix) 5. Phenanthroline solution (xxxi) 6. Stock iron solution 7. Standard iron solution ( 1 ml = 1/ug Fe) (xxxii) Procedure 1. Pipette 10, 20, 30 and 50 mL standard solution into 100mL conical flask. 2. Add 1 mL hydroxylamine solution and 1 mL sodium acetate solution to each flask. 3. Dilute each to about 75mL with distilled water. 4. Add 2 mL phenolphthalein solution to each flask. 5. Make up the contents of each flask exactly to 100 mL by adding distilled water and let stand for 10 minutes. 6. Take 50mL distilled water in another conical flask. 7. Repeat the steps 2 to 5 described above. 8. Measure the absorbance of each solution in spectrophotometer at 508 nm. Against the reference blank prepared by treating water as described in steps 6 and 7. Prepare a calibration graph taking meter reading on y-axis and concentration of iron on x – axis. 9. For visual comparison, pour the solution in 100 mL tall-form nessler tubes and keep them in a stand. 10. Mix the sample thoroughly and measure 50 mL in to a conical flask. 11. Add 2 mL concentrated Hydrochloric acid (HCL) and 1 mL hydroxylamine solution. Add a few glass beads and heat to boiling. To insure dissolution of all the iron, continue boiling until the volume is reduced to 15 to 20 mL. 12. Cool the flask to room temperature and transfer the solution to a 100 mL nessler tube. 13. Add 10mL Ammonium acetate buffer solution and 2 mL phenolphthalein solution and dilute to the 100ml mark with distilled water. 14. Mix thoroughly and allow at least 10 to 15 minutes for maximum colour development. 40
- 41. 15. Measure the absorbance of the solution in a 1 cm cell in a spectrophometer at 508 nm 16. Read off the concentration of iron (/ug Fe) from the calibration graph for the meter reading. 17. For visual comparison, match the colour of the sample with that of the standard prepared in steps 1 to 7 above. 18. The matching colour standard will give the concentration of iron in the sample (/ug Fe) Calculation Mg/L Iron (Fe) = /ug Fe = ml. of sample = Concentration of Fe in colour standard in /ug Spectrophotometer reading 41
- 42. Sample or description Volume of sample taken Concentration of Fe in a sample I /ug of matching colour standard or from graph Mg/L of Fe Result Sample No. or description Iron content mg/L (Fe) Discussion 42
- 43. B. DETERMINATION OF MANGANESE Aim The aim of the experiment is to determine the quantity of Manganese present in the given sample (s) by Per sulfate method Principle There is evidence that manganese occurs in surface waters both in suspension in the quadrivalent state and in the trivalent state in a relatively stable, soluble complex. Manganese occurs in domestic waste water, industrial effluents and receiving streams, but is generally unimportant except as it may enter a potable supply intake Per sulfate oxidation of soluble manganous compounds to from permanganate is carried out in the presence of Silver nitrate. The resulting colour is stable for at least 24 hours, if excess per sulfate is present and organic matter is absent Samples that have been exposed to air may give low results due to precipitation of manganese dioxide. Adding 1 drop of 30% hydrogen peroxide to the sample, after addition of the spectal reagent redissolves precipitated manganese. Apparatus 1. Colorimetric equipment : one of the following is required : a) Spectrophotometer, for use at 525nm, providing a light path of 1cm or longer. b) Nessler tubes, matched, 100ml tall form. 2. Glassware like conical flasks measuring cylinder and pipete Reagents 1. Special Reagent (xxxiii) 2. Ammonium per sulfate 3. Standard manganese solution (xxxiv) (1ml = 5/ug.Mn) 4. Hydrogen peroxide (H2O2) 30 % 43
- 44. Procedure 1. Take 50 ml of the sample in a conical flask. Add 50ml distilled water to it 2. Pipet 1, 2, 3, 4 and 8 ml of standard manganese solution to different conical flasks. Add 100 ml distilled water to all flasks. 3. Add 5ml special reagent to all the flasks. 4. Concentrate the solutions in all the flasks to about 90ml by boiling. 5. Add 1g Ammonium persulphate to all the flasks, bring to boiling and boil for I minute. 6. Remove all the flasks from the heat source and let stand for 1 minute. 7. Then cool the flasks under the tape water. 8. Dilute the contents in all the flasks to 100 ml with distilled water and mix. Pour contents into 100ml nessler tubes. 9. Match the colour of the sample with that of the colour standards. Note down the concentration of Mn in /ug. 10. If the spectrophotometer is used, one distilled water blank has to be prepared along with the colour standards. 11. Measure the absorbance of each solution in a 1cm cell at 252 nm against the reference blank prepared by treating distilled water. 12. Prepare a calibration graph taking meter reading on y-xis and concentration of manganese (in /ug) in the colour standards on x-axis. 13. Keep the sample in the spectrophotometer and note down the meter reading. 14. Read off from the graph the corresponding concentration of Manganese in /ug Calculation Mg/L Mn = /ug of Mn = Ml sample = 44
- 45. Observations Concentration of Mn in colour standards in /ug Spectrophotometer reading Sample No. or description Volume of sample taken Concentration of Mn in sample in /ug of matching colour standard or from graph Mg/L of Mn Result Sample No. or description Concn. Of Mn in mg/L Discussion 45
- 46. Experiment No.11 Date: DETERMINATION OF B.O.D (BIO CHEMICAL OXYGEN DEMAND) AND DISSOLVED OXYGEN A. DETERMINATION OF B.O.D Aim The aim of the experiment is to determine the amount of B.O.D exerted by the given sample(s) Principle The biochemical oxygen demand of sewage or polluted water is the amount of oxygen required for the biological decomposition of dissolved organic solids to occur under aerobic conditions and at a standardized time and temperature. Usually the time is taken as 5 days and the temperature 200 C. The B.O.D test is among the most important made in sanitary analysis to determine the polluting power, or strength of sewage, industrial wastes or polluted water. It serves as a measure of the amount of clean diluting water required for the successful disposal of sewage by dilution. The test has its widest application in measuring waste loadings to the treatment plants and evaluating the efficiency of such treatment systems. The test consists in taking the given sample in suitable concentrations in dilution water in B.O.D bottles. The two bottles are taken for each concentration and three concentrations are used for each sample. One set bottles are incubated in B.O.D indicator for 5 days at 200 C. the dissolved oxygen (initial) content (D1) in the other set of bottles will be determined immediately. At the end of 5 days the dissolved oxygen content (D2) in the other set of bottles is determined. Then mg/L B.O.D = (D1 – D2) P Where P = decimal fraction of sample used. D1 = dissolved oxygen of diluted sample (mg/L ) 15 minutes after preparation D2 = dissolved oxygen of diluted (mg/L) at the end of 5 days incubation 46
- 47. Among the three values of B.O.D obtained for a sample select that dilution showing a residual oxygen of at least 1mg/L and a depletion of at least 2 mg/L. If two or more dilutions are showing the same condition then select the B.O.D value obtained by that dilution in which the maximum dissolved oxygen depletion is obtained. Apparatus 1. B.O.D bottles 250 – 300mL capacity. 2. B.O.D indicator. 3. Burette 4. Pipette 5. Air compressor 6. Measuring cylinder etc. Reagents 1. Distilled water 2. Phosphate buffer solution (xx) 3. Magnesium sulphate solution (xxii) 4. Calcium chloride solution (xxiii) 5. Ferric chloride solution (xxiv) 6. Sodium sulphate solution (xxv) Procedure 1. Place the desired volume of distilled water in a 5 liter (usually about 3 liter of distilled water will be needed for each sample) 2. Add 1mL each phosphate buffer, magnesium sulphate solution calcium chloride solution and ferric chloride solution for every liter of distilled water 3. Saturate the dilution water in the flask by aerating with a supply of clean compressed air for at least 30 minutes. 4. Highly alkaline or acidic samples should be neutralized to pH 7 5. Destroy the chlorine residual in the sample by keeping the sample exposed to air for 1 – 2 hour or by adding a few ml of sodium sulphate solution. 6. take the sample in the required concentration. The following concentrations are suggested 47
- 48. Strong industrial waste : 0.1, 0.5 and 1 % Raw and settled sewage : 1.0, 2.5 and 5 % Oxidized effluents : 5, 12.5 and 25 % Polluted river water : 25, 50 and 100 % 7. Add the required quantity of sample (calculate for 650mL dilution water the required quantity of sample for a particular concentration) in to a 1000mL measuring cylinder. Add the dilution water up to the 650mL mark. 8. Mix the content in the measuring cylinder 9. Add the solutions into two B.O.D bottles one for incubation and the other for determination form of initial dissolved oxygen in the mixture. 10. Prepare in the same manner for other concentrations and for all the other samples. 11. Lastly filled dilution water alone into two B.O.D bottle. Keep one for incubation and other for determination of initial dissolved oxygen. 12. Place the set of bottles to be incubated in a B.O.D incubated for 5 days at 200 C. Care should be taken to maintain the water seal over the bottle through out the period of incubation. 13. Determine the initial dissolved oxygen content in the other set of bottles and note down the results. 14. Determine the dissolved oxygen content in the incubated bottles at the end of 5 days and note down the results. 15. Calculate the B.O.D of the given sample. Note: - The procedure for determining the dissolved oxygen content is same as described in part B of this experiment under “ Determination of dissolved oxygen” 48
- 49. Observations Concentration Dissolved oxygen content in mg/L B.O.D (5 days at 200 C) in mg/L B.O.D of the sample mg/L Sample No. or Description Initial (D1) Final (D2) Bottle No.1 D.O Value Bottle No. D.O Value D1-D2 B.O.D value Dilution Water Note:- B.O.D value in mg/L = { D1-D2 } P If concentration is 0.1% then P = 0.1 x 0.001 and so on 100 Calculation D1 = Initial dissolved oxygen = ________ mg/L D2 = Dissolved oxygen at the end of 5 days _______mg/L P = Decimal fraction of sample used _______ . . . mg/L of BOD = D1-D2 = P Result Sample No. or Description Mg/L 5 day BOD Discussion 49
- 50. B) DETERMINATION OF DISSOLVED OXYGEN Aim The aim of the experiment is to determine the quantity of dissolved oxygen present in the given sample(s) by using modified Winkler (Azide modification) method. Principle Dissolved oxygen (D.O) levels in natural and waste waters are depended on the physical, chemical and biochemical activities prevailing in the water bottle. The analysis of D.O is a key test in water pollution control activities and waste treatment process control. Improved by varies in technique and equipment and aided by instrumentation, the Winkler (or iodometric) test remains the most precise and reliable titrimetric procedure for D.O analysis. The test is based on in the addition of divalent manganese solution, followed by strong alkali to the water sample in a glass slopped bottle. D.O present in the sample rapidly oxides an equivalent amount of the dispersed divalent manganous hydroxide precipitate hydroxides of higher valency states. In the presents of iodide ions and up on acidification, the oxidized manganese reverts to the divalent state, with the liberation of iodine equivalent to the original D.O content in the sample. The iodine is the titrated with a standard solution of thiosulphate. Apparatus 1. 300mL capacity bottle with stopper 2. Burette 3. Pipettes etc. Reagents 1. Manganous sulphate solution (MnSo4. 4H2O) (viii) 2. Alkali – iodide Azide regent (ix) 3. Concn. Sulphuric acid (36N) 4. Starch indicator (x) 5. Standard sodium thiosulphate solution (0.025 N) (xi) 6. Standard potassium Dichromate solution (0.025N) (xii) 50
- 51. Procedure 1. Add 2 ml of manganous sulphate solution and 2 ml of alkali iodide acid reagent to the 300 ml sample taken in the bottle, well below the surface of the surface of the liquid. ( the pipette should dipped inside the sample while adding the above two reagents. 2. Stopper with care to exclude air bubbles and mix by inverting the bottle at least 15 minutes. 3. when the precipitates settles, living a clear supernate above the manganese hydroxide floc, shake again. 4. After 2 minutes of settling, carefully remove the stopper immediately add two ml conon sulphuric acid by allowing the acid to run down the neck of the bottle. 5. Restopper and mix by gentle inversion until dissolution is compleate. 6. Measure out 203 ml of the solution from the bottle to an Erlenmeyer flask. 7. Titrate with 0.025N sodium thiosulphate solution to a pale straw colour. 8. Add 1 – 2 ml starch solution and continue the titration to the first disappearance of the blue colour and note down the volume of sodium thiosulphate added (v) Calculation Because 1mL of 0.025N sodium thiosulphate solution is equivalent to 0.2mg of D.O each mL of sodium thiosulphate titrant used is equivalent to 1 mg./L D.O. When a volume equal to 200 mL of original sample is titrated. . . . mg./L of D.O = V = Result Sample No. or Description Temp. in 0 C D.O in mg./L Discussion 51
- 52. Experiment No.12 Date TEST FOR (B.COLI) COLIFORMS INDEX Aim The aim of this experiment is to determine the Most Probable Number (MNP) index of coliforms and E Coli or (B-Coli) organisms in the given sample(s) of water by the Multiple Tube fermentation Technique. Principle The coliform group of bacteria has been accepted as the indicator organism for faecal pollution in water. The E-Coli groups of organisms further coliforms the presence of faecal matter in the water tested. Since water of coliform organisms in water which indicates excretal contamination is of supreme importance. The coliform groups comprises all of the aerobic and facultative anaerobic gram negative, non spore forming rod – shaped bacteria which ferment lactose with gas formation within 48 hours at 350 C. The E-coli group belongs to the coliform group of faecal origin. They ferment lactose with gas formation within 24 hours at 440 C. The water is considered to be safe when these two organisms are absent. This should be conducted as a routine experiment in all the water treatment plants to check the efficiency of disinfections. Apparatus 1. Incubators 2. Test tubes 3. Platinum loop etc. Reagents 1. Macconkey broth (double strength) (xvi) 2. Brilliant green lactose by broth (xvii) 3. Ec medium (xviii) 52
- 53. Procedure 1. Collect the sample in sterilized bottles intended for bacteriological analysis 2. Prepare the sterilized media necessary bacteriological test and keep them ready in the test tubes containing Durham tubes. 3. Inoculate the sample in an exponential order i.e. 10, 1 and 0.1ml in five tubes if of Macconkey broth under complete aseptic conditions. 4. Incubate all the tubes at 35 0 C. 5. After 24 Hours examine the tubes for gas formation 6. The tubes containing the gas are marked positive and are taken out of the incubator for further analysis. The remaining tubes are further incubated at 350 C for another 24 hours. This is the presumptive test for coliform organisms 7. Take one or two loop full of the liquid from the positive Macconkey tubes and inoculate in Brilliant green lactose bile broth tubes and incubate the tubes for 24 hours at 350 C. Mark the tubes properly. 8. At the end of 24 hours examine the tubes for gas formation. The presents of gas confirms the presents of coliform organisms. The negative tubes further incubated for another 24 hours and then presents of absents of gas is noted. This is confirmatory test for coliform organisms. 9. The positive Macconkey tubes at the end of 24/48 hours are taken out. One or two loop full liquid is transferred to B.G.L.B. tubes for confirmatory test. The result is noted down. 10. One or two loop full of the liquid from the positive Macconkey tubes are transferred in to the sterilized EC mediums tubes. They are incubated for w24 hour at 440 C. 11. The gas production after 24 hours, confirms the presence of E-coli organism faceal colifirms. Calculations. Tabulate the result ( in the adjoining ) tabular column and find out the corresponding MPN index from the MPN table. Result. MPN of coliform organism = ________ in 100 mL of the sample MPN of E-coli or B-coli organism = ________ -do- Discussion 53
- 54. 55
- 55. APPENDIX PREPARATION OF REAGENTS AND MEDIA i) Phenolphthalein Indicator Solution Dissolve 5gm Phenolphthalein in 500 mL ethyl alcohol and add 500mL distilled water, then add 0.02N sodium hydroxide drop wise until a faint pink colour appears. ii) Methyl Orange Indicator Solution Dissolve 0.5gm Methyl orange in 1 Lt. of distilled water. Keep the stock solution in dark or in an amber coloured bottle iii) 0.02N Standard Sulphuric Acid Prepare stock solution approximately 0.1N by diluting 2.5ml concentrated sulphuric acid to 1Ltr. Dilute 200 ml of the 0.1N stock solution to 1Ltr. Co2 free distilled water. Standardize the 0.02 N acid against 0.02N sodium carbonate solution which has been prepared by dissolving 1.06g anhydrous Na2Co3 and diluting to the mark of a 1 liter volumetric flask. iv) 0.1N Sodium Thiosulphate Solution. Dissolve 25g sodium thiosulphate (Na2S2O3 5H2O) and dilute to 1liter with distilled water v) Ammonia buffer solution Dissolve 16.9g ammonium chloride (NH4Cl) in 143 ml concentrated ammonium hydroxide (NH4OH). Add 1.25g of Magnesium salt of EDTA and dilute to 250 ml, with distilled water or in the absence of the Magnesium salt of EDTA, dissolve 1.179g disodium salt of ethylenediamine tetra acetic acid dihydrate and 780mg. Mg.SO47H2O or 644mg. Mg Cl2O.6H2O in 50 ml distilled water. Add the solution to 16.9 g NH4Cl and 143ml concnt. NH4OH with mixing and dilute to 250ml with distilled water. 56
- 56. Do not store more than a months supply. Discard the buffer when 1 or 2 ml added to the sample fails to produce a pH of 10.0 ≠ 0.1 at the end point of titration. Keep the solution in a plastic or resistant glass container. vi) Erio Chrome Black T indicator Solution Mix 0.5g Eriochrome Black T dye with 4.5ml of hydroxylamine hydrochloride. Dissolve this mixture in 100 ml of 95% ethyl or isopropyl alcohol or mix 0.5g dye and 100g NaCl to prepare a dry powder mixture. vii) Standard EDTA Titrant (0.01N) Weigh 3.723g of the dry EDTA powder (ethylenediamine tetra acetate dihydrate also called ethylenediamine tetra acetic acid disodium salt) (Na2H2 C10H12O8N2) dissolve in distilled water an dilute to 1000ml viii) Manganous Sulphate Solution Dissolve 480g MnSO4. 4H2O or 400g MnSO4. 2H2O or 364g. MnSO4H2O in distilled water, filter and dilute to 1 liter. ix) Alkali Iodide – Azide Reagent Dissolve 500g sodium hydroxide ( NaOH) 700g Potassium hydroxide (KOH) and 135 g sodium iodide Nal or 150g potassium iodide KL in distilled water and dilute to 1 liter. To this solution add 10g sodium azide (NaN3) dissolved in 40ml distilled water. x) Starch Indicator Add 5g soluble starch to a little amount of distilled water and make it a suspension. Add the suspension to approximately 800ml of boiling water with stirring. Dilute to 1 liter allow to boil a few minutes, and let settle overnight. use the clean supernate. This solution may be preserved with 1.25g. salicylic acid per liter or by the addition of a few drops toluene. 57
- 57. xi) 0.025N sodium thiosulphate solution Dissolve 6.205g of sodium thiosulphate in freshly boiled and cooled distilled water and dilute it to 100ml. add 5ml chloroform or 0.4g NaOH/liter for preservation of the solution. Standardize this with 0.025N Potassium dichromate solution xii) 0.025N Potassium dichromate solution Dissolve 1.226g potassium dichromate and dilute to 1 liter Standardization of xi Dissolve approximately 2g KL in an Erlenmeyer flask with 100-150ml distilled water. Add 10ml 1+9 H2SO4, followed by exactly 20ml, 0.1N potassium dichromate solution. Place in the dark 4-5 minutes, dilute to approximately 400ml and titrate with 0.025N sodium thiosulphate, add starch towards the end of the titration. Exactly 20ml 0.025N thiosulphate will be consumed at the end of the titration Otherwise the thiosulphate solution should be suitably corrected. xiii) Potassium Chromate Indicator Dissolve 50g K2CrO4 in a little distilled water. Add silver nitrate solution until a definite red precipitate if formed. Allow to stand 12 hours, filter and dilute the filtrate to 1 liter distilled water. xiv) 0.0141N Silver Nitrate Solution. Dissolve 2.395 AgNO3 in distilled water and dilute to 1000 ml standardize against 0.0141N sodium chloride by means of the procedure described in determination of chlorides experiment. xv) 0.0141 N Sodium Chloride Solution Dissolve 842.1g sodium chloride in chloride free water and dilute 10 1000ml. 58
- 58. xvi) Orthotolodine Reagent Dissolve 1.35g orthotolodine dihydrochloride in 500ml distilled water. Add this solution with constant stirring to mixture of 350ml distilled water and 150ml concen, hydrochloric acid. Store the solution in brown bottle. Always use an automatic, dropping or safety pipet to measure the necessary volume. Avoid inhalation or exposure to the skin. xvii) MaC Conkey Broth (Double Strength) Commercial Sodium taurocholate or sodium tauroglycocholate : 10g Lactose : 20g Peptone : 40g Sodium chloride : 10g Distilled water : 1000ml Mix all the ingredients accepts the lactose, steam for 2 hour. Cool and keep in the refrigerator overnight. Add the lactose and when dissolved, filter through a good grade indicator. Add 1ml of a 1 % alcoholic solution of neutral red. 10ml of this medium is put into each of 15x1.5cm test tube. If 50ml quantities are to be tested, 50ml of the broth should be put into tubes or bottles of suitable size. Sterllise at 10psi (0.75kg/cm2 ) pressure for 15 minutes on three successive days. xviii) Brilliant Green Lactose Bile Broth Peptone : 10g Distilled water : 500ml Steam to dissolve and add the following solution Dehydrated ox-gall : 20g Distilled water : 200ml The ox-gall solution should be adjusted to a pH between 7 and 7.5. Make up with distilled water to approximately 975ml. Add 10g lactose adjust pH to 7.4 add 13ml of 3.1 % solution og Brilliant Green in distilled water and make it up to 1000ml. 59
- 59. Distribute in 5 mL quantities in fermentation tubes and sterilize in the autoclave at 10psi (0.75kg/cm2 ), pressure for 15 minutes on three successive days. The pH which may be determined by potentiometric method after sterillisation should be than 7.1 and not more than 7.4. xix) EC Medium Tryptose or trypti case : 20g Lactose : 5g Bile salts mixture or Bile salts No.3 :1.5g (KH2PO4) dipotassium hydrogen phosphate : 4g (KH2PO4) potassium hydrogen phosphate : 1.5g Sodium Chloride (NaCl) :5.0g Distilled water : 1 liter PH should be 6.9 after sterilization Prior to sterilization, dispense in termination tube with sufficient medium (10ml) to over the inverted Durham tubes at least partially after sterilization. xx) Preparation of Standard soap Solution 1. Make up a stock solution by shaking 80 to 100g of pure powdered castle soap with 1 liter or 80% grain alcohol, let stand overnight and decant. 2. prepare a standard calcium solution by dissolving exactly 0.5g of pure calcium carbonate in about 5ml of 1.3HCL Add about 40ml of boiled and cooled distilled water and add ammonium hydroxide until slightly alkaline to litmus. Make up to exactly 500ml with distilled water ( 1ml =1mg CaCo3) 3. Pipette 25ml of the standard calcium soap solution into a bottle and add 25ml of freshly boiled and cooled distilled water and titrate with the stock soap solution until a permanent lather is obtained and note ml of stock soap solution used = k 4. Find the lather factor of the stock soap solution 60
- 60. 5. Make a standard soap solution so that 1ml = 1mg of CaCO3. the amount of stock soap solution required to make 1 liter of standard soap solution may be obtained by using the following formula (K-lather factor) + 40 = ml stock solution required. xxi) Phosphate Buffer Solution Dissolve 8.5g potassium dihydrogen phosphate (KH2PO4), 21.7g dipotassium hydrogen phosphate (K2 HPO4), 33.4g disodium hydrogen phosphate hepta hydrate Na2HPO47H2O and 1.7g ammonium chloride (NH4 Cl) in about 500ml distilled water and dilute to 1 liter. The pH of this buffer should be e7.2 without further adjustment. Discard the reagent (or any of he following reagent ) if there is any sign of biological growth in the stock bottle. xxii) Magnesium Sulphate Solution Dissolve 22.5g MgSO4 7H2O in distilled water and dilute to 1 liter. xxiii) Calcium Chloride Solution Dissolve 27.5g anhydrous CaCl2 in distilled water and dilute to 1 liter xxiv) Ferric Chloride Solution Dissolve 0.25g FeCl3. 6H2O in distilled water and dilute to 1liter. xxv) Sodium Sulphate Solution (0.25N) Dissolve 1.575g anhydrous Na2SO3 in 1000ml distilled water. This solution is not stable. Prepare daily. xxvi) Hydrochloric Acid (HCL) Concentrated, containing less than 0.00005% iron. xxvii) Hydroxylamine Solution Dissolve 10g. hydroxylamine hydrochloride salt in 100ml distilled water. 61
- 61. xxviii) Ammonium Acetate Buffer Solution Dissolve 250g ammonium acetate in 150 ml distilled water. Add 700ml concentrated acetic acid. Prepare a new reference standard with each buffer preparation. xxix) Sodium Acetate Solution Dissolve 200g sodium acetate in 800ml distilled water. xxx) Phenanthroline Solution Dissolve 100mg 1,10- Phenanthroline monohydrate C12H8N2.H2O, in 100ml distilled water by stirring and heating to 800 C. Do not boil, discard the solution if it darkens. Heating is unnecessary if two drops of concentrated HCL are added to the distilled water. xxxi) Stock Iron Solution Add slowly 20 lm concentrated H2 SO4 to 50 ml distilled water and dissolve 1.404g Ferrous Ammonium sulphate [Fe(NH4 )26H2]. Add 0.1Pottassium permanganate drop wise until faint pink colour persists. Dilute to 1000ml with iron free distilled water and mix. Each 1 ml = 200/ug Fe. xxxii) Standard Iron Solution Pipette 5ml stock solution into 1liter volumetric flask and dilute to the mark with iron free distilled water. 1ml = 1/ug Fe xxxiii) Special Reagent Dissolve 75g mercuric sulphate, (HgSO4) in 400 ml concentrated (HnO3) nitric acid and 200ml distilled water. Add 200 ml 85% Phosphoric acid (H3PO4) and 35mg silver nitrate. Dilute the cooled solution to 1 liter. xxxiv) Standard Manganese Solution Prepare a 0.1NKMnO4 (Potassium permanganate) solution by dissolving 3.2g of KMnO4 in distilled water and making up to 1 liter. Age for several weeks in sunlight or 62
- 62. heat for several hours near the boiling point. Then filter through a fritted glass filter crucible and standardize against oxalate solution. Calculate the volume of the solution necessary to prepare 1 liter of solution of such strength that 1 ml = 50/ug Mn as follows ML KMnO4 = 4.55 Normality of KMnO4 To this volume add 2 to 3 ml concentrated H2SO4 and sodium bisulphate solution (10g sodium bisulphate plus 100ml distilled water) drop wise stirring until the permanganate colour disappears. Boil to remove excess SO2. cool and dilute to 100 ml with distilled water. Dilute 10ml of this solution to 100ml with distilled water whenever required. The strength of this solution will be 1 ml = 5/ug. xxxv) Methyl Red Indicator Solution Dissolve 100mg methyl red sodium salt in distilled water and dilute to 100ml xxxvi) Barium Chloride Solution Dissolve 100g (BaCl2. 2H2O) barium chloride in 1 liter distilled water. Filter through quality filter paper (What man filter paper No. 42) 1 ml of the reagent is capable of precipitating about 40mg SO4. xxxvii Silver Nitrate Nitric Acid Reagent Dissolve 8.5g AgNO3 and 0.5ml concen. HNO3 (Nitric acid) in 500ml distilled water. xxxviii) Standard Iodine Solution, 0.025N Dissolve 20 to 25g potassium iodide (Kl) in a liter water and 3.2g Iodine. After the iodine has dissolved dilute to 1000ml and standardize against 0.025N sodium thiosulphate, using starch solution as indicator. 63
- 63. APPENDIX II STANDARD FOR DRINKING WATER Requirement Acceptable Cause for Rejection + Physical 1 Turbidity ( turbidity units) 2.5 10 2 Colour units on platinum cobalt scale 5 25 3 Taste and odour Not objectionable Chemical 4 PH 7 to 8.5 Less than 6.5 or grater than 9.2 5 Total solids mg/L 500 1500 6 Total hardness ( as CaCo3) mg/L 200 600 7 Calcium (as Ca) mg./L 75 200 8 Magnesium (as Mg) mg/L 30 150 9 Iron (as Fe) mg/L 0.1 1 10 Manganese (as Mn) mg/L 0.05 0.5 11 Copper (as Cu) mg/L 0.05 1.5 12 Zinc (as Zn) mg/L 5.0 15 13 chlorides (as cl) mg/L 200 1000 14 Sulphates (as SO4) mg/L 200 400 15 Phenolic substances (as phenol ) mg/L 0.001 0.002 16 Fluorides as(F)mg/L 1.0 2.0 17 Nitrates (as NO2) mg/L 45 45 Toxic substances 18 Arsenic (as As) mg/L 0.05 0.05 19 Chromium (as hexavalent) mg/L 0.05 0.05 20 Cyanides (as Cn) mg/L 0.05 0.05 21 Lead (as Pb) mg/L 0. 0.1 22 Selenium (as Se) mg/L 0.01 0.01 Radio Activity 23 Alpha emitters / uc/mL 10-9 10-9 24 Betta Emitters /uc/mL 10-8 10-8 Bacteriological quality 25 MPN Index of Coliform Bacteria Should be zero or less than one 10 per ++ 100 ml 64
- 64. + Figures in excess of the permissive while not acceptable may still be to-lerated in the absence of alternative and better sources, but up to the limits designated, above which the supply will not be acceptable. + + Occasionally, the samples may show an MPN index 3 to 10 per 100 mL provided this does not occur in consecutive samples. When consecutive samples show an MPN Index exceeding 8 per 100 mL additional samples should be collected promptly from the sampling point and examined with out delay. This should be done daily until the MPN index of samples collected on two successive days is within the acceptable limits. If necessary, samples should also be taken from several other points such as the service reservoirs, distribution systems, pumping stations and treatment plant and examined for coliforms. In addition, the operation of all treatment processes should be checked and remedial measures taken if necessary. When the results obtained over a period of one month are considered, not more than 10% of the samples examined during the period should have shown an MPN index of coliforms grater than 1 per 100 mL. 65
- 65. APPENDIX –III MPN TABLE No. of Tubes giving positive reactions out of MPN Index per 100mL 95% confidence limits 5 of 10 mL each 5 of 1 mL each 5 of 0.1mL each Lower Upper 0 0 0 < 1 - - 0 0 1 2 1.0 10 0 1 0 2 1.0 10 0 2 0 4 1.0 13 1 0 0 2 1.0 11 1 0 1 4 1.0 15 1 1 0 4 1.0 15 1 1 1 6 2.0 18 1 2 0 6 2.0 18 2 0 0 4 1.0 17 2 0 1 7 2.0 20 2 1 0 7 2.0 21 2 1 1 9 3.0 24 2 2 0 9 3.0 25 2 3 0 12 5.0 29 3 0 0 8 3.0 24 3 0 1 11 4.0 29 3 1 0 11 4.0 29 3 1 1 14 6.0 35 3 2 0 14 6.0 35 3 2 0 17 7.0 40 4 0 0 13 5.0 38 4 0 1 17 7.0 45 4 1 0 17 7.0 46 4 1 1 21 9.0 55 4 1 2 26 12 63 4 2 0 22 9.0 56 4 2 1 26 12 65 4 3 0 27 12 67 4 3 1 33 15 77 4 4 0 34 16 80 5 0 0 23 9.0 86 5 0 1 30 10 110 5 0 2 40 20 140 5 1 0 30 10 120 5 1 1 50 20 150 5 1 2 60 30 180 5 2 0 50 20 170 5 2 1 70 30 210 5 2 2 90 40 250 5 3 0 80 30 250 66
- 66. 5 3 1 110 40 300 5 3 2 140 60 360 5 3 3 170 80 410 5 4 0 130 50 390 5 4 1 170 70 480 5 4 2 220 100 580 5 4 3 280 120 690 5 4 4 350 160 820 5 5 0 240 100 940 5 5 1 300 100 1800 5 5 2 300 500 300 5 5 3 900 600 2900 5 5 4 > 1600 600 5300 5 5 5 1600 - - Note:- 1. If instead of portions of 10.1 and 0.1mL, a combination of 100, 10 and 1mL is used then the MPN is recorded as 0.1 times the value given in the table 2. For 1, 0.1 and 0.01 combination then 10 times the value given in the table should be used. 3. For 0.1, 0.01 and 0.001 combinations, 100 times the value given in the table should be used. 67
- 67. Sl.No. Date Name of Expeiment Page No. Marks 1 DETERMINATION OF SOLIDS 2 DETERMINATION OF TURBIDITY 3 DETERMINATION OF ALKALINITY 4 DETERMINATION OF HARDNESS 5 DETERMINATION OF pH 6 DETERMINATION OF CHLORIDES 7 DETERMINATION OF CHLORIDES 8 DETERMINATION OF AVAILABLE CHLORINE IN BLEACHING POWDER AND TEST FOR RESIDUAL CHLORINE 9 DETERMINATION OF SULPHATES AND SULPHIDES 10 DETERMINATION OF IRON AND MANGANESE 11 DETERMINATION OF B.O.D (BIO CHEMICAL OXYGEN DEMAND) AND DISSOLVED OXYGEN 12 TEST FOR (B.COLI) COLIFORMS INDEX 68
- 68. 69
- 69. 70
- 70. SampleNo.orDescription Dateandtimeofobservation Dateandtimeofincubation Volume of sample inoculated in mL TotalNoofpositivetubesafterconfirmatorytest MPN Test for Remarks 10 10 10 10 10 1 1 1 1 1 0.1 0.1 0.1 0.1 0.1 Coliform organisms 24 Hrs Presumptive test 48hrs Presumptive test 24 hrs confirmatory test 48 hr confirmatory test E-coli Or B-coli 24 hr confirmatory test 71
- 71. 72
