Diffusion final
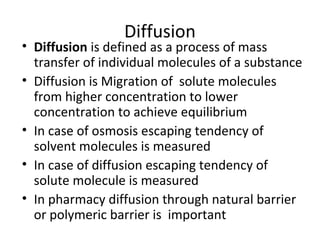
- 1. Diffusion • Diffusion is defined as a process of mass transfer of individual molecules of a substance • Diffusion is Migration of solute molecules from higher concentration to lower concentration to achieve equilibrium • In case of osmosis escaping tendency of solvent molecules is measured • In case of diffusion escaping tendency of solute molecule is measured • In pharmacy diffusion through natural barrier or polymeric barrier is important
- 2. • membrane is film separating the phases which may be porous or non porous • Diffusant or permeant orpenetrant is The material that undergoes the transport by passive diffusion Application • Controlled and sustained release follows diffusion controlled • Molecular weight of polymer can be estimated • Transport of drug from GIT can be predicted through diffusion studies
- 3. • Diffusion of drugs into tissues and their excretion through kidney can be studied through diffusion • Dialysis,micronisation,ultrafiltration,haemodia lysis,osmosis use the principle of diffusion
- 4. STEADY STATE DIFFUSION • At Steady state - conditions do not vary with time • In case of diffusion mass transfer remains constant with time OR mass transfer takes place at constant rate through the study and diffusion process is not allowed to attain equilibrium • If condition vary with time then the system is under unsteady state
- 5. Transport cell • Transport cell is used to study the diffusion • Which consists of donor and receptor compartment separated by membrane • Permeant dissolved in solvent and placed in donor compartment • Vehicle is placed in receptor compartment • The permeant get transported in to receptor comportment through membrane • At steady state mass transfer remains constant
- 6. SINK CONDITION • It is the state in which the concentration in the receptor compartment is maintained at lower level compared to its concentration in the donor compartment • This can be maintained by connecting receptor compartment to a large reservoir from which solution is reticulated • It is easy to maintain sink condition than steady state condition due to maintaining constant gradient in donor compartment is difficult
- 7. Flux • Rate of mass transfer (dM/dt) expressed as of flux(J) • Flux (J) is rate of mass transfer across unit surface area of a barrier and mathematically expressed as: J ≡ atoms / area / time 1 dM dM = change in mass of material, g J= S = surface area.cm2 S dt d t = change in time.sec Units for flux are g.cm -2sec -1 OR kg .meter -2sec -1 Flux is always positive quantity because it increases continuously during process
- 8. Fick’s I law • Fick’s first law states that the flux is directly proportional to the concentration gradient J ≡ atoms / area / time ∝ concentration gradient dc OR dc Negative sign indicates J∝ J = − D ...( 2) a decrease in concentration dx dx But flux is positive quantity flux in steady state flow dc=change in conc. of material g/cm 3. D=diffusion coefficient of a penetrant, cm/sec2. Dx=change in the distance, cm. 1 dM dc J= J = −D 1 dM dc S dt dx J= = −D Combining equation and i.e. S dt dx dM dc ...(3) Eqn 3 explains Rate of We get = −DS mass transfer as per fick’s first law dt dx D is effected by temperature, pressure etc hence it is not constant it is coefficient
- 9. Fick’s I law Diffusion coefficient/ diffusivity No. of atoms dM dc crossing area A = − DS Cross-sectional area per unit time dt dx Concentration gradient Mass transport is down the concentration gradient Flow direction A
- 10. Application of fick’s first law • Used to explain drug diffusion across biomembranes with desirable parameters • Applied in the design of sustained and controlled release systems
- 11. Fick’s Second Law ; Non-steady state Diffusion It explains the change in conc. at definite location with respect to x , y and z axes(or direction) Fick’s second law states that the change in y J y concentration With time in a particular region is proportional to the change In the concentration Jx gradient at that point of time Jz x z ∆c The concentration ∂c i.e. ∆t changes with time due to ∆J change in amount or flux ∂J i.e. of diffusing ∆x molecules with in the x direction
- 12. • The relationship can be expressed w.r.t -x ,y and z as: = − ∂J ∂c ∂c ∂J ∂c ∂J =− =− ∂t ∂x ∂t ∂y ∂t ∂z Partial derivatives notation used due to concentration is a function of both x or y or z and t dc dc dc J = −D J = −D J = −D dx dy dz Differentiating above equation w.r.t x ,y and z respectively ∂J ∂C 2 ∂J ∂ 2C ∂J ∂ 2C = −D 2 = −D = −D 2 ∂x ∂x ∂y ∂ y2 ∂z ∂z substituting for ∂C , ∂C and ∂C in above equation for ∂J , ∂J and ∂J ∂t ∂t ∂t ∂x ∂y ∂z ∂C ∂C 2 ∂C ∂ 2C ∂C ∂ 2C = −D 2 = −D = −D 2 ∂t ∂x ∂t ∂ y2 ∂t ∂z
- 13. ∂C ∂ C ∂ C ∂ C 2 2 2 = −D 2 + + 2 ∂t ∂ x ∂y 2 ∂z Fick’s second law refers to change in concentration of diffusant with time at any distance x i.e. non steady state flow
- 14. DIFFUSION CONTROLLED RELEASE HIGUCHI’S EQUATION • Sustained and controlled release of a drug form a table has been obtained by incorporating the drug in insoluble matrix such as plastic ,resin, wax and fatty alcohol • In this matrix model ,outside layer of the drug is exposed to the bathing solution • Then the drug diffuses out of the matrix • The rate of dissolution of drug particle within the matrix must be faster than that of diffusion rate of drug leaving the matrix
- 15. The rate of release of drugs dispersed in an inert matrix system has been derived by higuchi dM Cs = C0 dx − ........(1) dt 2 where dM = change in the amount of drug released per unit time dx = change in the thickness of the zone of matrix that has been depleted of drug C 0 = total amount of drug in unit volume in the matrix Cs = saturated conc of the drug in the matrix
- 16. Dm Cs From diffusion theory dM = dt........( 2 ) x Where Dm is diffusion coefficient in the matrix Equating eqn 1 and 2 ,integrating and solving for x gives M = [ Cs Dm ( 2C0 − C s ) t ] 1 2 When the amount of drug in excess of saturation concentration that is Co>Cs M = [ C s Dm 2C0t ] 2 ......( 3) 1 Eqn 3 indicates that the amount drug released is a function of square root time M = kt 1 OR 2
- 17. Methods and procedures • Two types • A) horizontal transport cell wurester cell Viles chein permeation cell • B) vertical transport cell Aquair and weiner diffusion cell biber and rhodes cell franz diffusion cell
- 18. Horizontal Transport Cell wurester cell Receptor and donor compartment made of pyrex glass material Animal or human skin acts as semi permeable cell and barrier may be supported on a perforated plate Drug sample solution taken in donor compartment and solvent in the receptor compartment Whole set up placed in constant temperature bath to maintain the temp of 37±0.2 The liquid in receptor stirred by using magnetic beads to obtain
- 19. vertical Transport Cell Viles chein skin permeation cell Receptor and donor compartment made of pyrex glass or glass or plexi glass material Animal or human skin acts as semi permeable cell This system used for as in vitro models for drug absorption and used to test drug diffusion from ointments ,transdermal patches etc Drug sample solution taken in donor compartment and solvent in the receptor compartment Whole set up placed in constant The liquid in receptor stirred by using temperature bath to maintain the magnetic beads to obtain uniform distribution temp of 37±0.2
- 20. Horizontal Transport Cell Aquair and weiner diffusion cell Receptor and donor compartment made of pyrex glass or plastic material Animal or human skin acts as semi permeable cell and barrier may be supported on a perforated plate Drug sample solution taken in upper compartment and solvent in the lower compartment Whole set up placed in constant temperature bath to maintain the temp of 37±0.2 The liquid in receptor stirred by using magnetic beads to obtain
- 21. Horizontal Transport Cell biber and rhodes cell This is three compartment cell Two Receptor and one donor compartment Synthetic or isolated biological membrane can be used Drug sample solution allowed to diffuse from two donor compartment to inner receptor compartment liquid in receptor stirred by using magnetic beads to obtain uniform distribution
- 22. Horizontal Transport Cell scheuplein cell Receptor and donor compartment made of pyrex glass material Animal or human skin acts as semi permeable cell and barrier may be supported on a perforated plate Drug sample solution taken in donor compartment and solvent in the receptor compartment Whole set up placed in constant temperature bath to maintain the temp of 37±0.2 The liquid in receptor stirred by using magnetic beads to obtain uniform distribution
- 23. Horizontal Transport Cell franz diffusion cell modified version of different cell for in-vitro studies Excised human cell membrane acts as semi permeable membrane Animal or human skin acts as semi permeable cell and barrier may be supported on a perforated plate Drug sample solution filled in donor compartment and solvent in the receptor compartment Whole set up placed in constant temperature bath to maintain the The liquid in receptor stirred by using temp of 37±0.2 magnetic beads to obtain uniform distribution
- 24. Transport across GI tract • Most of drugs ,when administered ,have to pass through GI membrane to reach blood • The structure and nature of GI tract decide the transport of drugs • These barrier are highly complex structure composed of lipids ,proteins , lipoproteins and polysaccharides and lipoidal in nature
- 25. Polar heads Fluid Mosaic love water Model of the & dissolve. cell membrane Non-polar tails hide from water. Carbohydrate cell markers Proteins
- 27. Types of diffusion • Passive diffusion • Active transport • Facilitated diffusion • pinocytosis
- 39. Answer any Four Questions Each question carries 5 marks 1) List out the method to determine interfacial tension explain any one method 2) Write a note on factors influencing rate of reaction 3) What are chelates give its application 4) What are adsorption isotherms explain different types 5) Explain chemical degradation by oxidation 6) Write a note on size distribution curves 7) Explain-how particle sizes are expressed 8) Define and differentiate order and molecularity of a reaction