Case record...Friedreich ataxia
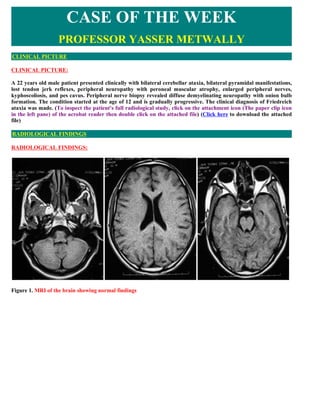
- 1. CASE OF THE WEEK PROFESSOR YASSER METWALLY CLINICAL PICTURE CLINICAL PICTURE: A 22 years old male patient presented clinically with bilateral cerebellar ataxia, bilateral pyramidal manifestations, lost tendon jerk reflexes, peripheral neuropathy with peroneal muscular atrophy, enlarged peripheral nerves, kyphoscoliosis, and pes cavus. Peripheral nerve biopsy revealed diffuse demyelinating neuropathy with onion bulb formation. The condition started at the age of 12 and is gradually progressive. The clinical diagnosis of Friedreich ataxia was made. (To inspect the patient's full radiological study, click on the attachment icon (The paper clip icon in the left pane) of the acrobat reader then double click on the attached file) (Click here to download the attached file) RADIOLOGICAL FINDINGS RADIOLOGICAL FINDINGS: Figure 1. MRI of the brain showing normal findings
- 2. Figure 2. MRI T2 (A,B) and MRI T1 (C) showing marked atrophy of the uppermost part of the cervical spinal cord Figure 3. MRI T1 (A) and MRI T2 (B) showing marked atrophy of the uppermost part of the cervical spinal cord
- 3. Figure 4. MRI T2 images showings cervical cord atrophy, thinning with reduced anteroposterior diameter. Notice the hyperintense line in posterior portion of cord. The thinned spinal cord is seen lying on the posterior wall of spinal canal with increased signal intensity in its posterior and lateral compartments. The anterior subarachnoid space is enlarged. The intramedullary signal changes reflect loss of myelinated fibers and gliosis. Pathophysiology of Friedreich ataxia The major pathophysiologic finding in FA is a "dying back phenomena" of axons, beginning in the periphery with ultimate loss of neurons and a secondary gliosis. The primary sites of these changes are the spinal cord and spinal roots. There is a loss of large myelinated axons in peripheral nerves, which increases with age and disease duration. Unmyelinated fibers in sensory roots and peripheral sensory nerves are spared. The posterior columns, corticospinal, ventral, and lateral spinocerebellar tracts all show demyelination and depletion of large myelinated nerve fibers to differing extents. This is accompanied by a fibrous gliosis that does not replace the bulk of the lost fibers. Overall, the spinal cord becomes thin and the anteroposterior (AP) and transverse diameters of the thoracic cord are reduced. The dorsal spinal ganglia show shrinkage and eventual disappearance of neurons associated with proliferation of capsular cells. The posterior column degeneration accounts for the loss of position and vibration sense and the sensory ataxia. The loss of large neurons in the sensory ganglia causes extinction of tendon reflexes. Large neurons of the dorsal root ganglia, especially lumbosacral, and nerve cells in Clarke's column are reduced in number. The posterior roots become thin. The dentate nuclei exhibit mild to moderate neuronal loss and the middle and superior cerebellar peduncles are reduced in size. There is patchy loss of Purkinje cells in the superior vermis of the cerebellum and of neurons in corresponding portions of the inferior olivary nuclei. There are mild degenerative changes in the pontine and medullary nuclei and optic tracts. The cerebellar ataxia is explained by loss of the lateral and ventral spinocerebellar tracts, involvement of Clarke's column, the dentate nucleus, superior vermis, and dentatorubral pathways. The corticospinal tracts are relatively spared down to the level of the cervicomedullary junction. Beyond this point, the corticospinal tracts are severely degenerated, which becomes progressively more severe moving down the spinal cord. This explains the common finding of bilateral extensor plantar responses and weakness late in the disease. Loss of cells in the nuclei of cranial nerves VIII, X, and XII results in facial weakness, speech, and swallowing difficulty. Myocardial muscle fibers also show degeneration and are replaced by macrophages and fibroblasts. Essentially, chronic interstitial myocarditis occurs with hypertrophy of cardiac muscle fibers; fibers become hypertrophied and lose their striations. This is followed by swelling and vacuolation and finally interstitial fibrosis. The nuclei appear hyperchromatic and occasionally vacuolated. The cytoplasm appears granular with frequent lipofuscin depositions. Kyphoscoliosis is likely, secondary to spinal muscular imbalance.
- 4. Figure 5. Friedreich Ataxia Histologic Findings in Friedreich ataxia A cross-section through the lower cervical cord clearly shows loss of myelinated fibers of the dorsal columns and the corticospinal tracts (Weil stain). Milder involvement of spinocerebellar tracts is also present. The affected tracts show compact fibrillary gliosis (hematoxylin and eosin [H&E]) but no breakdown products or macrophages, reflecting the very slow rate of degeneration and death of fibers. The dorsal spinal ganglia show shrinkage and eventual disappearance of neurons associated with proliferation of capsular cells (H&E). The posterior roots are nearly devoid of large myelinated fibers. Within the thoracic spinal cord, degeneration and loss of cells of the Clarke column is apparent. Figure 6. Friedreich Ataxia, Spinal cord Comment on the radiology of the previously reported case The decreased anteroposterior diameter of the spinal cord at the upper cervical region confirms that atrophy of the upper cervical part of the spinal cord is a characteristic feature of Friedreich’s ataxia, as opposed to other forms of corticocerebellar and cerebellar-brainstem atrophy [3-6]. This had been indicated on the basis of subjective evaluation in two previous studies [201,204]. No direct pathologic correlation of the intramedullary signal abnormalities is available. However, the sensitivity of MR imaging to degeneration of white matter tracts in the brain and spinal cord after stroke or in degenerative diseases of the CNS - that is manifested on the MRI T2 images as hyperintense lines- has been cited in several reports [211-214]. Because of the substantial similarities between the intramedullary signal abnormality pattern that is found in the reported patient and the distribution of demyelination and gliosis of white matter tracts in the histopathologic pictures of the spinal cord in cases of Friedreich’s ataxia [200], we think it reasonable to assume that the MR appearance could reflect these pathologic findings. Obviously, the intramedullary signal abnormality pattern we found is not exclusive to Friedreich’s ataxia and can be observed in subacute combined degeneration, tabes dorsalis, wallerian degeneration, and AIDS myelopathy. In these conditions, however, associated clinical and laboratory findings usually allow the correct diagnosis. Detection of signal changes in the white matter tracts of the spinal cord of patients with Friedreich’s ataxia could be an index of severity or progression of the disease and in this respect it is more useful than cord atrophy. The
- 5. association between the extent of intramedullary signal changes and the chronicity and severity of disease was not examined in the reported patient. Although this analysis could be informative, it requires quantitation of the signal changes in the white matter tracts and evaluation of the thoracolumbar spine, which were not done in the reported patient. Noteworthy is the fact that we found intramedullary signal changes only in patients with Friedreich’s ataxia. No such findings were seen in any of the patients with corticocerebellar or cerebellar-brainstem atrophy in the author experience. Thus, it appears that evaluation of the cervical spinal cord for intramedullary signal changes might be useful for differential diagnosis in patients with progressive ataxia of uncertain clinical type. In a broad sense, our results confirm that MR examination of the cervical spinal cord is more informative than examination of the brain in patients with Friedreich’s ataxia. Although spinal cord atrophy and intramedullary signal changes theoretically could be searched for in the thoracic spinal cord of patients with Friedreich’s’ ataxia [200], focusing on the cervical spinal cord is recommended because it usually allows concurrent evaluation of the brainstem and the cerebellum. This may help in the differential diagnosis with corticocerebellar and cerebellar- brainstem atrophies. In conclusion, MR imaging of the cervical spinal cord can show thinning of the cord and intramedullary signal changes consistent with degeneration of white matter tracts in the lateral and posterior columns of patients with Friedreich’s ataxia. These MR findings might be helpful for differential diagnosis in patients with progressive ataxia of uncertain clinical type. DIAGNOSIS: DIAGNOSIS: FRIEDREICH'S ATAXIA DISCUSSION DISCUSSION: Hereditary ataxias are a heterogeneous group of neurological disorders. Outstanding contributions by Holmes [1], Greenfield [2] and Harding [3] established a syndromic framework for these diseases, and remarkable progress in the field now permits a definitive diagnosis in 60 to 70 percent of hereditary ataxia patients. Although an increasing number of genetic tests are becoming available for the known ataxia genes, phenotypic variability and clinical overlap have hampered development of a useful clinical algorithm for the differential diagnosis of ataxic patients. Furthermore, some patients with hereditary ataxia may have no family history of ataxia. The diagnosis of genetic ataxia in apparently sporadic cases is especially difficult, as a variety of nongenetic causes could be underlying the manifestation of the ataxic syndrome. Most of the genetic ataxias are adult-onset disorders for which there are no effective treatments, and it is not yet possible to offer satisfactory estimates concerning age of onset or prognosis in potentially affected relatives. The results of DNA testing however, may have dramatic ethical, social and legal impact on the life of test subjects and their families. For these reasons, careful genetic and psychological counseling is required for the patients who undergo genetic testing. It should also be noted that some genetic ataxias are treatable and correct diagnosis for these disorders should not be missed. In this article, the author reviews the clinical features and current genetic understanding of inherited ataxias. The readers may also refer to recent reviews of hereditary ataxias [4] and dominant hereditary ataxias [6,7,116] . As ataxia commonly presents in patients without family history, we will discuss the clinical approach to differential diagnosis of hereditary and non-hereditary conditions. AUTOSOMAL DOMINANT ATAXIA Spinocerebellar ataxias Autosomal dominant spinocerebellar ataxias (ADCA; see Table 1) have been clinically classified into three syndromes (ADCA-I, II and III), based on the presence of cerebellar ataxia in combination with other symptomatology [8,9]. ADCA-I is defined by a cerebellar syndrome variably accompanied by ophthalmoplegia, pyramidal and extrapyramidal signs, dementia and other neurological disorders, ADCAII is defined by a cerebellar syndrome associated with pigmentary retinopathy and ADCA-III is defined by pure ataxia without other neurologic features. While this classification scheme is still useful, more recent definition of diseases by specific genetic loci or based on individual families (defined by specific Spinocerebellar ataxias (SCA) loci), only roughly correlates with Harding's classification. SCA 7 is the only genetically defined disorder in the ADCA-II group. ADCA-I would
- 6. classically include SCAs 1, 2, 3, 4, 12, 13 and 17, but each of these disorders may have purely cerebellar features early. Most patients with SCA 5, 6, 8, 10, 11, 14 and 16 would be classified with ADCA-III but others develop extracerebellar neurological abnormalities consistent with ADCA-I. Thus the SCA terminology based on genetic analysis is more commonly presented today. Table 1. Autosomal dominant cerebellar ataxia (ADCA) Distribution % of Type of mutation/Gene Normal Pathogenic Disease Locus product alleles alleles (number of total Db families) SCA ADCA I SCA-1 6p22-p23 CAG expansion/ataxin-1 6-44 40-81 worldwide 5-15% C 12q23- SCA-2 CAG expansion/ataxin-2 14-31 34-59 Cuba, worldwide 5-15% C 24.1 SCA- 14q24.3- Portugal, CAG expansion/ataxin-3 14-33 56-200 30-50% C 3/MJD q32.1 worldwide USA, Japan (7 SCA-4 16q22.1 unknown ? N families) CAG expansion/5 UTR of USA, India (2 SCA-12 5p31-p33 17-28 55-78 rare R PPP2R2B families) 19q13.3- SCA-13 unknown France (1 family) rare R 13.4 Impure CAG SCA-17 6q27 27-44 47-63 Japan, Germany rare R expansion/TBPa ADCA II SCA-7 3p12-p13 CAG expansion/ataxin-7 7-19 37-300 worldwide ? C CTG expansion/3 UTR SCA-8 13q21 15-91 107-127 ? C region of SCA8 ADCA III SCA-5 11cen unknown USA (1 family) rare N CAG SCA-6 19p13.1 4-20 21-33 worldwide 5-10% C expansion/CACNL1A4a ATTCT expansion/intron 9 SCA-10 22q13 10-22 800-4500 Mexico rare C Hh-E4a 15q14- England (1 SCA-11 unknown rare N q21.3 family) 19q13.4- SCA-14 unknown Japan (1 family) rare N qter 8q22.1- SCA-16 unknown Japan rare N q24.1 Episodic Ataxia EA type Missense 12p13 worldwide R 1 mutations/CACNL1A4a EA type Missense 19p13 worldwide R 2 mutations/CACNL1A4a DRPLA [b] D: Diagnosis available=Commercially (C), Research (R), Non-available (N) [a] TBP (TATA binding proteins); CACNL1A4 (1A-voltage-gated calcium channel subunit); Hh-E4 (human homologous of mouse E4 gene) Many of the SCAs (1, 2, 3, 6, 7, 17 and dentatorubro-pallidoluysian atrophy (DRPLA)) are caused by an expansion of CAG repeats in their respective genes. In these disorders, the mutation is an expansion of a naturally occurring
- 7. CAG repeat in the protein coding region of a specific protein. This leads to an abnormal expanded series of glutamines being translated in that specific protein. The expansion is unstable over generations leading to progressive lengthening of the repeat. Disease results when the repeat reaches a specific pathologic range, and further expansion leads to progressively earlier age of onset. Thus, in each of these diseases the CAG repeat size correlates inversely with the age of onset. The common pathogenic mechanism of these diseases involves a gain of toxic function [10], and neuropathological studies in “polyglutamine” diseases reveal the presence of neuronal nuclear protein inclusion [11,12], which contain the polyglutamine-containing protein, specific interacting proteins, and proteins from the ubiquitin-degradation pathway [10]. These aggregates do not appear to be responsible for the disease in SCA1 [13], although their direct role in other “polyglutamine” diseases is unclear. The pathogenicity of the mutant protein in these diseases may involve defective “protein clearance,” sequestration of transcription co- activators and co-repressors, and/or other gain of function mechanisms [14]. The mutations for SCA8 and SCA12 are expansions of a CAG/CTG repeat in untranslated regions of their respective genes. Whether the trinucleotide expansion found in SCA8 is a causative mutation or a polymorphism in linkage disequilibrium is still a matter of debate (see SCA8, below). Individuals with SCA10 have a large expansion of an intronic ATTCT pentanucleotide repeat. The pathogenic mechanisms of these SCAs remain to be investigated. SCAs4, 5, 11, 13, 14 and 16 have been mapped, but their mutations have not been identified. SCAs1, 2, 3, 6, 7 and 17 are more frequent than others SCAs, and a few SCA types have been recognized only in a single family. The incidence of the different types of SCA around the world is variable, and known SCAs constitute about 60–80% of the families with ADCA (Table 1). SCA1 The SCA1 gene is located on chromosome 6p, linked to the HLA gene complex [15]. SCA1 was cloned, and the CAG repeat expansion identified, in 1993 [16]. The number of CAG repeats in normal and pathological alleles of SCA1 are shown in Table 1. The progression of SCA1 has a pattern common to most of the ADCA [17]: typical onset with a cerebellar syndrome (i.e. broad-based gait, limb ataxia, progressive dysarthria), hand tremor, and oculomotor signs including intermittent nystagmus, slowing in saccadic velocity followed by limitation of up-gaze. The bulbar and upper motor neurons signs may include lingual fasciculations, noncerebellar dysphagia and dysarthria, brisk deep tendon reflexes and increased tone (spasticity). Later in the disease, this constellations of symptoms becomes more severe with coughing, overt choking, sensory loss, mild peripheral neuropathy and extensor plantar responses. In SCA1, extrapyramidal signs may be evident by the time the patient becomes wheelchair bounded. The phenotype of the disease appears to be relatively homogeneous within and between families. Variability observed in age of onset and progression appears to be attributable to the size of the CAG repeat expansion [18,19]. Bradykinesia and parkinsonism, which may be prominent features in SCA3/MJD, have not been observed in SCA1. Cognitive decline does not appear to be severe in SCA1. Neuroimaging studies typically show atrophy of cerebellar cortex, vermis and brainstem. SCA2 The SCA2 gene was mapped by linkage analysis of a homogeneous population of Holguin province (Cuba), to chromosome 12q24 [20], and later cloned independently by three groups [21–23]. Clinical features of the disease include slow saccades, kinetic or postural tremor and decrease of muscle tone and tendon reflexes. Variable incidence of dystonia, chorea and dementia has also been recognized. Nonspecific cerebellar or pontocerebellar atrophy has been observed on MRI. Nerve conduction studies show evidence of axonal sensory-motor neuropathy. SCA3/MJD (Machado-Joseph disease) Originally described in Portuguese families and named the “Azorean disease” [24], SCA3 is now recognized worldwide. The mutation in the SCA3 gene, localized to 14q31.1, is an expanded CAG repeat in a novel protein, designated ataxin-3 [25]. Variable phenotypes are found in SCA3[26,27]; in addition to cerebellar ataxia and ophthalmoplegia, early-onset disease can present with spasticity, dystonia and an akinetic syndrome, whereas late- onset SCA3 presents mainly with minimal extrapyramidal symptomatology. The extrapyramidal signs are a characteristic, but not exclusive, feature of the SCA3 phenotype [27]. There is an inverse correlation between the age of onset and the expanded CAG repeat length. The severity of the disease as well as the presence of some symptoms (i.e. peripheral neuropathy) may depend on the length of the expansion, with short and very large pathological alleles lacking neuropathy [28]. SCA4
- 8. A five-generation Utah kindred with an autosomal dominant, late-onset spinocerebellar ataxia with the invariable presence of prominent axonal sensory neuropathy and normal eye movements demonstrated linkage to chromosome 16q22.1 [29]. Six Japanese families with pure cerebellar ataxia without sensory neuropathy also showed linkage to this locus [30,31]. SCA5 SCA5 was assigned to chromosome 11 in a single family descending from the grandparents of President Abraham Lincoln [32]. In this family, 56 individuals had cerebellar ataxia with onset at 10 to 68 years. Anticipation is evident, and progression of the disease is slow. SCA5 does not affect life span, probably because severe bulbar disease is generally absent. A second, apparently unrelated, SCA5 family of French origin showed a similar phenotype with marked global cerebellar atrophy similar to SCA6 on MRI [33]. SCA6 SCA6 classically presents with pure cerebellar ataxia although patients with a prolonged disease course may show other clinical features including dystonic postures, involuntary movements, and abnormalities in tendon reflexes [34]. Patients with SCA6, especially those with late onset disease, may present without a family history. The diagnosis of SCA6 should be considered in sporadic cases with horizontal and oblique gaze nystagmus and an abnormal vestibulo-ocular reflex without other ocular movement abnormalities or those with pure cerebellar atrophy [34,35]. Some phenotypic overlap with the allelic disease, EA-2 (see the section of Episodic Ataxia), has been noted [36,37]. Brain MRI shows atrophy of the cerebellum, and in some patients, pons, middle cerebellar peduncles and red nucleus [34,38,39]. The SCA6 mutation is a small CAG repeat expansion within the 1A-voltage-dependent calcium channel subunit (CACNL1A4) gene [40]. The number of CAGs in mutant alleles ranges 20 to 30 while normal alleles are 4 to 17 CAGs (Table 1) [40,41]. An allele with 19 CAGs has been reported in a Japanese patient with ataxia [42]. Although the age of onset inversely correlates with the expanded repeat size in different families, the expanded repeat is usually stably transmitted within each family [41,43,44]. In rare cases, however, mild expansions have been reported [45,46]. Sisters homozygous for (CAG)25/25 showed a similar age of onset but different progression rate, suggesting pathogenic contribution of factors other than the repeat size [47]. Homozygous cases suggested a gene dosage effect on the age of onset [36,48–50]. Anticipation in the absence of changes in the CAG repeat size has been described in French [51], Japanese [41] and Taiwanese kindreds. Although the expansion size of the CAG repeat falls into the normal ranges of other SCAs, the polyglutamine tract in a densely packed membrane protein may allow the ? 1A subunits with small expansions to aggregate [52,53]. A key question is whether the SCA6 mutation causes disease by making the ? 1A subunit toxic, or it does so by perturbing the gating of P/Q-type channels [54]. To reconcile these two different mechanisms, it has been postulated that protein interactions altered by the expanded polyglutamine tract might cause gating abnormalities [55]. SCA7 SCA7 is characterized by progressive ataxia and pigmentary maculopathy with anticipation and is the only disease classified as an ADCA-II. These features are accompanied by other clinical features including pyramidal and extrapyramidal signs, ophthalmoplegia, dementia, hypoacusis, hypotonia and auditory hallucinations [56]. Slowing of voluntary and involuntary saccades is also an early sign of SCA7 [57]. Specific juvenile and infantile forms of SCA7 have been defined. Juvenile SCA7 occurs with maternal and paternal transmission, whereas the infantile form occurs only with paternal transmission. The infantile form shows severe hypotonia, cerebral and cerebellar atrophy, early visual loss, congestive heart failure, and patent ductus arteriosus [58]. SCA7 is caused by an expansion of a CAG repeat in the SCA7 (ataxin-7) gene on chromosome 3p [59,60]. The normal range is 7 to 19 CAG repeats, and pathogenic alleles range from 37 to approximately 306 repeats. The expansion size correlates with the severity of the disease, and inversely correlates with the age of onset [61,62]. Alleles with 28 to 36 repeats are intermediate alleles that are prone to further expansion, accounting for de novo mutation cases [63,64]. Different disease-associated haplotypes segregated among SCA7 kindreds, suggesting multiple origins of the mutation [63]. Paternal transmission is underrepresented, and when it does occur is associated with significant intergenerational expansion of the CAG repeat [65]. The expanded CAG repeat in male germline is biased toward massive size increases, potentially leading to putative embryonic lethality or dysfunctional sperm [66]. The available data suggest that the mutant ataxin-7 protein is pathogenic due to a gain of toxic function by the polyglutamine expansion [67– 70]. SCA8
- 9. SCA8 presents with slowly progressive ataxia with marked cerebellar atrophy [71]. The disease is variably associated with pyramidal and cognitive dysfunctions [72]. SCA8 patients have an expansion of a CTG repeat on chromosome 13q21 to the pathogenic range of 100 to 152 repeats, while normal individuals have 15 to 91 CTGs [73]. Affected individuals usually inherit an expanded allele from their mothers. SCA8 alleles show an instability bias toward expansion with maternal transmission, whereas paternal transmissions mostly result in smaller alleles, which appear to be due to contraction of the expanded alleles to the normal range (<100 CTGs) in the sperm [71,74,75]. Variable penetrance has been identified in SCA8 pedigrees [71,76]. There has been no evidence of correlation between the repeat size and the age of onset. Haplotype analyses showed multiple origins for the SCA8 expansions [72]. The SCA8 CTG repeat is preceded by a polymorphic but stable CTA tract with the configuration (CTA)1-21(CTG) n in the 3? untranslated region of the SCA8 gene. Many SCA patients have expanded alleles with interruptions by cryptic repeats (CCG, CTA, CTC, CCA or CTT), which may have newly arisen or changed from generation to generation [74]. SCA8 contains no open reading frame, but the most 5? exon is transcribed through the first exon of another gene, KLHL1, which is transcribed in the opposite orientation. This raises a possibility that the SCA8 transcript is an endogenous antisense RNA for KLHL1. KLHL1 encodes a cytoplasmic protein that may play a role in organizing the actin cytoskeleton of the brain cells. Both SCA8 and KLHL1 are primarily expressed in specific brain tissues affected by SCA8 [77]. Other gain-of-function mechanisms involving the mutant SCA8 transcript have not been investigated, but by no means excluded. The major controversy in SCA8 stems from a lack of absolute specificity of the CTG repeat expansion for SCA8, which has raised a possibility that CTG expansions at the SCA8 locus represent a rare polymorphism rather than the disease-causing mutation [78,79]. Alleles greater than 100 CTG repeats at the SCA8 locus were also found in 1.2% (14 of 1,120) of major psychosis patients and 0.7% (5 of 710) of normal control subjects who had no family history of ataxia [80]. SCA9 (vacant) SCA10 The clinical phenotype of SCA10 is characterized by a combination of relatively pure cerebellar ataxia and epilepsy. Ataxia is progressive and often leads to total disability. Seizures are generalized motor seizures, complex partial seizures, or both. Some patients show mild cognitive dysfunction, sensory polyneuropathy, and/or soft corticospinal tract signs [81]. Anticipation is conspicuous in some families but subtle or absent in others. The disease has, so far, been confined to families of Mexican descent. The SCA10 mutation is a very large expansion of the ATTCT pentanucleotide repeat located in intron 9 of the E46L (SCA10) gene on chromosome 22q13 [49]. Normal alleles show 10 to 21 ATTCTs while affected individuals have 800–4500 repeats. An inverse correlation exists between the size of the expanded allele and the age of onset. The mechanism of the disease remains unknown. SCA11 SCA11, clinically defined as an ADCA-III, has been mapped to chromosome 15q in a single British family [78]. SCA12 SCA12 is a rare ADCA characterized by adult onset ataxia, upper body tremor, hyperreflexia with bilateral extensor plantar responses, paucity of movements and dementia [82,83]. The clinical picture includes oculomotor abnormalities (i.e. broken pursuit, nystagmus, slow saccades). The disease is slowly progressive and in the late stage psychiatric symptoms, including depression, anxiety, or delusions may appear in some affected members. Brain MRI shows cerebellar and cerebral cortical atrophy. There is no evidence of anticipation. The mutation of SCA12 is a CAG repeat expansion in the 5? untranslated region of the PPP2R2B gene on chromosome 5p31-p33 [84]. Normal alleles have 17 to 28 CAG repeats while the pathologic alleles have 55 to 78 repeats. The disease has been described in two kindreds, one from US and the other from India [85,87]. It has been postulated that the expansion of the CAG repeat up-regulate PPP2R2B expression. SCA13 A French family presented with slowly progressive childhood-onset cerebellar gait ataxia, dysarthria, moderate mental retardation and mild motor developmental delays with, in some cases, nystagmus and pyramidal signs. Brain MRI showed moderate cerebellar and pontine atrophy. A genome wide search showed linkage to chromosome
- 10. 19q13.3-q13.4 [86]. SCA14 A Japanese three-generation family with ADCA mapped to a locus on chromosome 19q13.4-qter [86]. The family members with late onset (>39 years old) exhibited pure cerebellar ataxia, whereas those with an early onset (<27 years old) showed intermittent axial myoclonus followed by ataxia. Other neurological signs were sparse, and neuroimaging studies revealed isolated cerebellar atrophy. SCA15 (vacant) SCA16 This is a pure cerebellar ataxia with head tremor, and thus a form of ADCA III [88]. Although head tremor, which is distinct from tittubation, has been described in SCA2, SCA7, and SCA12, it is rare for all SCA types. Age of onset ranges 20 to 66 years, and anticipation was not apparent. Brain MRI showed cerebellar atrophy without involving brainstem. The SCA16 locus showed linkage to markers on chromosome 8q22.1-q24.1 [88]. SCA17 An expansion of CAG repeats of the TATA-binding protein (TBP) gene was initially reported in a Japanese patient with sporadic cerebellar ataxia and intellectual deterioration associated with de novo expansion. The expanded allele consisted of impure CAG repeats encoding 63 glutamines, and the de novo expansion involved partial duplication of the CAG repeat [89]. In two German families, four patients showed expanded CAG repeats in TBP gene coding for 50 to 55 polyglutamines while normal subjects had alleles ranging from 27 to 44 glutamines [90]. Several additional Japanese families with phenotypes resembling Huntington's disease or DRPLA also showed SCA17 CAG expansions [91]. Other SCAs Anecdotally there are many ADCA families who are not linked to known genetic loci and who have no distinctive phenotype. In one such French family, SCAs 1, 2, 3, 6, 7, 8, and 12 were excluded by mutation analysis and SCAs 4, 5, 10, 11, 13, 14 by lod score less than ?2. Affected members of this French family showed gait ataxia, akinesia, dysarthria, hyporeflexia and anticipation [92]. There are likely to be many similar families who have not yet been characterized or reported. DRPLA DRPLA is a relatively frequent disease in Japan with an incidence of 0.2-0.7 per 100,000. A few isolated families have been recognized worldwide [92]. The DRPLA gene, on the chromosome 12p, was cloned in 1994 [94,95]. Early onset of the disease includes ataxia, myoclonus epilepsy and progression to dementia, later onset disease can present with cerebellar symptoms, psychiatric symptoms, seizures [93,96] and choreoathetotic abnormal movements with a “Huntington-like” phenotype [97]. Age of onset and severity correlate with the length of the CAG expansion. The mutation in the DRPLA gene has also been found in the Haw River Syndrome, which has some different clinical characteristics from DRPLA [98]. Episodic ataxia (EA) In contrast to SCAs, these rare diseases present with episodic ataxic attacks of variable frequency and duration, with no symptoms between attacks. Additional characteristic signs are associated with the different types of EA. EA type 1 (EA1) EA1 is caused by mutations in a potassium channel gene, KCNA1, on chromosome 12p [99]. The onset is between childhood and adolescence. Besides episodic ataxia, clinical manifestations are myokymia around the eyes, lips or fingers. Brief attacks of ataxia and dysarthria lasting seconds are precipitated by exercise or startle. EA type 2 (EA2)
- 11. Clinical manifestations of EA2 consist of mild to severe intermittent cerebellar dysfunction associated with oculomotor abnormalities, such as interictal nystagmus and diplopia, and migraine. Symptoms can be present for minutes to a few days. Episodes are provoked by stress and exercise but not startle. Like familial hemiplegic migraine (FHM) and spinocerebellar ataxia type 6 (SCA6) EA2 is associated with mutations in the gene encoding the ?-1A subunit of the calcium channel. SCA6 is caused by a CAG repeat expansion [40], while point mutations in the CACNA1A gene cause EA2 and FHM [100]. Missense mutations usually cause FHM, and non-sense or splice site mutations cause EA2 [101]. However, this rule has many exceptions [102] and EA2, FHM, and SCA6 may represent a clinical continuum of the distinct mutations in CACNA1A[103]. Moreover, an identical mutation can give various clinical expressions in a same family. Paroxysmal symptoms could arise from reversible channel dysfunction while progressive neurodegeneration could reflect a toxic gain of function. The latter appears to be a general phenomenon in polyglutamine diseases (see SCA 6), but it is not known how point mutations in CACNA1A are associated in some cases with neuronal degeneration [103]. EA2 may respond to the treatment with acetazolamide [104,105]. EA with paroxysmal choreoathetosis and spasticity The onset of this disorder is at 2–15 years, with ataxia, dystonia and headaches [105]. Episodes last for a few minutes with a daily to yearly frequency. Spastic paraparesis can persist between the attacks. Episodes are provoked by fatigue, stress, exercise, and alcohol. AUTOSOMAL RECESSIVE ATAXIA WITH CHILDHOOD OR JUVENILE ONSET Friedreich's ataxia Friedreich's ataxia (FRDA), described in 1863 by Nicholaus Friedreich and characterized in detail by Harding [8], is the most frequent autosomal recessive ataxia. It has an incidence in whites of 1:30,000 [106], but has not been documented among Sub-Saharan Africans, American Indians, and peoples from China, Japan, and Southeast Asia [107,108]. FRDA is responsible for 30 to 40 percent of autosomal recessive ataxia among whites. The disease is a progressive spinocerebellar degeneration with typical onset before the age of 25 years, especially during childhood or puberty (7–14 y) [8]. The degeneration of the posterior columns of the spinal cord, afferent cerebellar pathways and selected nuclei of the cerebellum gives rise to an ataxic gait, classically followed by dysarthria, upper limb ataxia, distal sensory loss, decreased vibration and position sense, and absence of reflexes. Scoliosis and pes cavus also can develop. The full picture of the disease often includes hypertrophic cardiomyopathy [109], impaired glucose tolerance or diabetes mellitus, and occasionally hearing loss or optic atrophy [110]. In early onset cases, FRDA may need to be differentiated from adrenomyeloneuropathy by very long chain fatty acid (VLCFA) levels, Refsum's disease by serum phytanic acid levels, and vitamin E deficiency by vitamin E levels. In some cases, dominant ataxias and Charcot-Marie-Tooth disease join in the differential diagnoses. Neuropathological changes in FRDA include degeneration of the dorsal root ganglia, Clarke's columns, posterior columns, corticospinal/spinocerebellar tracts, cerebellum, pons and medulla [111]. Nerve conduction studies show selective loss of sensory nerve action potentials. The FRDA/X25 gene contains seven exons spanning 80 kb on chromosome 9q13, and encodes a 210 amino acid protein called frataxin [112,129]. Most FRDA patients have an expansion of the trinucleotide GAA in the first intron of the FRDA/X25 gene [114]. Normal alleles contain 6 to 28 GAA repeats, while affected individuals have 60 to 1800 (Table 2). Point mutations in the FRDA gene (instead of the GAA expansion) are found in about two to four percent of the cases. Compound heterozygous patients with one expanded GAA repeat allele and one point mutation are more likely to show variant clinical features such as optic atrophy or retained reflexes than patients carrying homozygous GAA expansions [115]. Among patients with 2 alleles carrying expanded GAA repeats, there is an inverse correlation between the size of the GAA expansion and the age of onset, severity of the disease and the occurrence of cardiomyopathy and diabetes. Patients with the “late onset” (after 25 years old) variant (LOFA) and those with retained reflexes (FARR) are also caused by the FRDA gene mutation [5,117] most commonly associated with modestly expanded alleles (i.e. <400 repeats) [127]. Genetic heterogeneity in FRDA has been proposed, and a second locus (FRDA2) has recently been identified [118]. Table 2. Hereditary ataxia according to the age of onset Age of onset Diagnostic clues p.i.a Locus/gene Diagnosis Early onset (connatal or childhood) Main Congenital Ataxia Syndromes
- 12. Joubert's syndrome abnormal eye movements AR ? Clinic Gillespie's syndrome partial anyridia AR ? Clinic Behr's syndrome optic atrophy AR several Clinic Marinesco-Sjögren's cataracts, hypogonadism AR ? Clinic syndrome Main Metabolic Ataxias Urea cycle's disorders urinary organic acids AR several Clinic/Biochem/Genet Aminoacidurias urinary amino acids AR several Clinic/Biochem/Genet Disorders of serum pyruvate/lactate AR several Clinic/Biochem/Genet pyruvate/lactate Mitochondrial encephalomyopathies MELAS, MERRF, etc Mb several Clinic/Biochem/Genet Peroxisomal disorders infantile Refsun syndrome AR several Clinic/Biochem/Genet Disorders of DNA repair skin sensitivity, α- Ataxia telangiectasia AR 11q22-23/ATM Clinic/Genet fetoprotein, cancer Xeroderma pigmentosum skin sensitivity, cancer AR several Clinic/Genet skin sensitivity, Cockayne's syndrome AR several Clinic/Genet dysmorphology skin sensitivity, brittle Trichothiodystrophy AR several Clinic/Genet hair Intermediate onset (childhood or juvenile) sensorimotor loss, Friedreich's ataxia - FRDA AR 9q13/frataxin Clinic/Genet cardiac, skeletal Early onset ataxia with early onset, retained AR several? Clinic/Genet retained reflexes - EOARR reflexes Isolated vitamin E low serum levels of AR 8q13/TTP1 Clinic/Biochem/Genet deficiency - AVED vitamin E Spastic ataxia of hypermyelation retinal- Charlevoix-Saguenay - AR 13q11/sacsin Clinic/Genet nerve fibers SACS Recessive ataxia with ocular ocular apraxia, AR 9q13/HIT/Zn-finger Clinic/Genet motor apraxia -AOA neuropathy Progressive myoclonus myoclonus, ataxia, AR ? Clinic ataxia - PMA seizures Progressive myoclonus Unverricht-Lundborg ARc 21q22.3/cystatin B Clinic/Genet epilepsy - PMEc syndrome Abetalipoproteinemia abetalipoproteinemia AR 4q22-24/MTP Clinic/Biochem/Genet Hypobetalipoproteinemia hypobetalipoproteinemia AD 2p24, 3p22 Clinic/Biochem Niemman-Pick disease type 18q11/NPC1 (95%) sphingomyelin lipidosis AR d Clinic/Biochem/Genet C galactosylceramide Krabbe's disease AR galactocerebrosidase Clinic/Biochem/Genet lipidosis Hexosaminidase hexosaminidase deficit AR hexA and hexAB Clinic/Biochem/Genet deficiencies Late Onset (juvenile or adult) Autosomal dominant Several (see Table SCAs AD Clinic/Genet cerebellar ataxia - ADCA 1) Episodic ataxia EA-1, EA-2 and EPCA AD channelopaties Clinic/Genet Von Hippel-Lindau cerebellar AD 3p25/VHL Clinic/Genet syndrome hemangioblastomas Cerebellar ataxia with hypogonadism, ataxia AR? ? Clinic hypogonadism Mitochondrial multisystemic several Clinic/Biochem/Genet [a] pattern of inheritance (ie, AR: autosomal recessive).
- 13. [b] Mitochondrial diseases due to defective mitochondrial genes have maternal inheritance, whereas mitochondrial diseases due to nuclear genes have AR/AD. [c] Other clinical forms of PME (ie, the neuronal ceroid lipofuscinoses, Lafora disease, type I sialidosis and MERRF) are associated with defects in various genes. [d] A causative second gene, NPC2, has not been identified. A fascinating concurrence of yeast and human genetics [119–124] led to the proposal that frataxin is involved in the homeostasis (probably the transport) of mitochondrial iron. Accumulation of iron inside the mitochondria of FRDA cells could lead to increased oxidative stress by free radical production from hydrogen peroxide and hypersensitivity to the oxidative stress due to mitochondrial dysfunction. Deficiencies found in mitochondrial/cytoplasmic aconitase as well as in the respiratory chain complexes I, II and III in FRDA patients [123,125] are consistent with this hypothesis. Moreover, primary vitamin E deficiency (see later), a specific disease associated with a defect in a physiological free radical scavenger, clinically resembles FRDA. Interestingly, all of the enzymes with defective activity in FRDA mitochondria have iron-sulfur (Fe-S) clusters, at the active sites. These moieties are highly sensitive to free radical damage. An alternative or complementary hypothesis is that a primary activity of frataxin is related to the synthesis of the Fe-S cluster [112]. In any case, these findings led to the proposal that antioxidants could be useful for the treatment of FRDA. Idebenone (a free-radical scavenger, analogue of coenzyme Q10) may ameliorate and/or to revert the cardiac hypertrophy in FRDA patients [126]. This drug is currently being studied in clinical trials. Although iron chelation by desferroxamine should decrease serum and cytoplasmic iron levels, it may decrease aconitase activity, which may be detrimental to the cellular function. In addition, whether chelation can alter mitochondrial iron levels without toxic side effects is unknown. A recently developed mouse model of FRDA [113] raises a hope for providing a powerful means to explore many of these hypotheses and potential treatments. Other than the potential therapeutic effect of antioxidants on cardiomyopathy in FRDA, the treatment remains supportive and symptomatic. Physiotherapy and orthopedic management (prosthesis or surgery) are used either to preserve or to correct progressive deformations of the spine and feet. It is important to provide appropriate management of cardiac functions, glucose metabolism and secondary medical problems such as aspiration pneumonia. Early-onset cerebellar ataxia with retained reflexes This disease was considered by Harding [128], to be a syndrome distinct from FRDA; however, genetic mapping of the early-onset cerebellar ataxia with retained reflexes (EOCARR) locus is necessary before it can be recognized as an independent genetic entity [127]. Onset is before age 20 with a clinical picture similar to FRDA but with retained reflexes, low incidence of sensory neuropathy, less reduction of joint position sense, and absence of skeletal abnormalities, optic neuropathy, cardiomyopathy or diabetes [127]. Exclusion of FRDA by DNA testing is essential since some patients with the EOCARR phenotype have GAA expansions in the frataxin gene [127,128]. Moreover, a branch of a recently described EOCARR family has the late onset form of FRDA while another branch has EOCARR phenotype [127]. In EOCARR with very early onset, differential diagnosis with ataxia telangiectasia, mitochondrial disorders, adrenoleukodystrophy and lysosomal storage diseases, may be necessary. Ataxia with vitamin E deficiency Ataxia with vitamin E deficiency (AVED) is a rare treatable disorder with a phenotype that resembles FRDA. In a patient with a clinical diagnosis of FRDA and a negative genetic result for FRDA, serum vitamin E levels should be determined as patients with AVED have very low levels of vitamin E (<5 ?g/ml). Age of onset is between childhood and young adulthood associated with slowly progressive gait and limb ataxia, loss of deep tendon reflexes, and vibratory and proprioceptive loss, followed by progressive ophthalmoplegia, dysarthria, extensor plantar responses and muscle weakness. The disorder is caused by a defect in the TTP1 gene [129] on chromosome 8q13, encoding the ?-tocopherol transfer protein [130] responsible for the transfer of ?-tocopherol to the circulating lipoproteins. Early onset and severe forms of the disease are caused by truncating mutations or missense mutations in conserved amino acids [131]. However, DNA testing for the AVED mutations is not commercially available. Early replacement therapy may prevent progression of the disease [132,133]. Several diseases that lead to secondary vitamin E deficiency (ie, by affecting the absorption and/or transport of fat
- 14. and vitamin E) also cause a slowly progressive spinocerebellar syndrome (ie, ataxia, hypo/areflexia, limitation in upward gaze, proprioceptive loss, etc). The main causes of secondary vitamin E deficiency are: severe malnutrition, cystic fibrosis, cholestatic liver disease, extensive intestinal resection, hypolipoproteinemia, abetalipoproteinemia (Bassen-Kornzweig disease, see below) and Marinesco-Sj?gren syndrome [134]. Some of these patients have an associated retinopathy. Abetalipoproteinemia (Bassen-Kornzweig disease) This rare autosomal recessive disorder results in secondary Vitamin E deficiency and a phenotype similar to that observed of acquired vitamin E deficiency consisting of spinocerebellar degeneration, peripheral neuropathy and retinitis pigmentosa [135]. Acanthocytosis (“thorny” red blood cells) is observed in Bassen-Kornzweig disease (BKD) and other neurodegenerative diseases, such as neuroacanthocytosis and the Mcleod syndrome [136]. The onset of BKD is in childhood, with steatorrhoea and fat malabsorption features resembling celiac disease. Mutations in the microsomal triglyceride transfer protein (MTP) gene on 4q22-24 [137,138] account the defects in some BKD patients. This protein transports triglyceride, cholesterol ester and phospholipids across various lipid membrane systems. Vitamin E replacement therapy prevents further progression of the neurological symptoms. Hypobetalipoproteinemia is a genetically distinct disorder with a similar clinical presentation to BKD. Recessive ataxia with ocular motor apraxia This disease was originally described as mimicking ataxia-telangiectasia [139]. A recent study of 22 patients from Portugal suggests that this entity is more frequent than initially postulated [140]. A similar clinical entity, Early- onset cerebellar ataxia with hypoalbuminemia (EOCA-HA), has been described in Japanese patients [141]. The ataxia with ocular motor apraxia (AOA1) locus is located on 9q13 [142]. AOA1 and EOCA-HA are allelic diseases as shown by the recent cloning of the gene encoding aprataxin, a new HIT/Zn-finger protein [141,143]. AOA is manifested by onset in childhood of gait imbalance, followed by dysarthria, cerebellar ataxia, characteristic ocular apraxia [144] and peripheral neuropathy. Severe motor neuropathy dominates the clinical picture in the advanced phases of the disease, in which ocular apraxia evolves into external ophthalmoplegia [140]. Proposed diagnostic criteria for AOA are: autosomal recessive transmission, childhood onset, a clinical presentation of cerebellar ataxia, ocular apraxia, and areflexia, followed by a later appearance of peripheral neuropathy. Although dystonia, scoliosis, and pes cavus may be associated, mental retardation, telangiectasia, and immunodeficiency do not occur in AOA. AOA can resemble SCA2 clinically, but the inheritance pattern should distinguish these disorders (see above). Spastic ataxia of charlevoix-saguenay Originally described in the region of Charlevoix-Saguenay at Quebec, autosomol recessive spastic ataxia of charlevoix-saguenay (ARSACS) is an autosomal recessive disease with early onset manifesting initially with gait ataxia, which is followed by progressive axonal neuropathy and spastic paraplegia. Fundus examination of optic reveals hypermyelination of the nerve fiber layer [145]. Intelligence is normal although lowered IQ has been recognized in some cases. MRI studies show cerebellar atrophy. The gene, mapped to chromosome 13q11 [145], has a simple giant exon (12,794 bp) encoding a protein named sacsin, a putative chaperone highly expressed in the granular cell layer of the cerebellum [146]. The same spastic ataxia of charlevoix-saguenay (SACS) locus has recently been linked to the hereditary ataxia in two Turkish families [147], raising a possibility that ARSACS could be more common than previously expected. Disorders associated with defective DNA repair Ataxia telangiectasia This is an autosomal recessive disorder with an incidence of 1:100,000. Gait ataxia is a prominent sign in these patients, first observed during infancy or childhood [148]. The disease has neurological (ocular apraxia, slow horizontal saccades, cerebellar syndrome, dystonic postures, chorea, tics or jerks, dysphagia and choking, as well as peripheral neuropathy with the progression of the disease), dermatological (telangiectasia, premature senile keratosis and graying of the hair), and immunological (atrophy of thymus and lymphoid tissues, lymphopenia, low levels of immunoglubulins) components. DNA sensitivity is the basis of all of these manifestations as well as the occurrence of cancer (mostly lymphoma and leukemia) in about twenty percent of ataxia telangiectasia (AT) patients. Elevated serum ?-fetoprotein levels are observed in about 90 to 95 percent of AT patients and non-random chromosomal aberrations (ie chromosomes 7 and 14) are found in lymphocytes. Survival after the age of 30 is rare. The disease is caused by mutations in the gene ataxia telangiectasia, mutated (ATM) on 11q22-23 [149]. ATM encodes a protein with serine/threonine kinase activity from the family of the phosphatidyl inositol-3-kinases [150].
- 15. Conversely, a mutation in the gene hMRE11, involved in double strand break repair, has been found in an ataxic patient with an AT-like phenotype [151]. Xeroderma pigmentosum, Cockayne's syndrome and trichothiodystrophy These autosomal recessive diseases combine extreme skin photosensitivity, ataxia and occasionally other neurological manifestations, such as seizures, chorea, dystonia, peripheral neuropathy, mental retardation, deafness and dementia [152]. No commercially available diagnostic tests are available at present. Xeroderma pigmentosum (XP) is associated with a strong predisposition to cutaneous cancers, squamous cell carcinomas and melanomas, and less frequently with nervous system neoplasms. The age of onset is from childhood to adult. The disease is heterogeneous with eight complementation groups, XP-A through XP-G, and XP-V (XP- Variant) [153]. XP affects transcription-coupled repair and global genomic repair. Not all the XP groups have neurological signs [152,154]. Cockayne's syndrome (CS) is characterized by marked growth retardation, moderate skin photosensitivity and premature aging. The patients have short stature with disproportionally long arms and legs, and large hands and feet. Abnormal developmental features are associated with a characteristic facial appearance consisting of microcephaly, thin nose, deep set eyes, progressive lack of subcutaneous fat and prognathism. Neurological signs may include mental retardation, retinal and cochlear degeneration, demyelinating neuropathy and ataxia. Severe atrophy of the white matter is observed in these patients. No increased risk of cancer is recognized in CS patients. The genes associated with CS are CS-A, CS-B, XP-B, XP-D and XP-G. Groups XP-B, XP-D and XP-G correspond to genes whose mutations could produce either XP or CS phenotypes [155,156]. According to the severity and genetic basis, two forms of CS have been described, both representing mutations in the gene CS-B on chromosome 10q11: i) a classical severe infantile variant, and ii) the cerebro-oculo-facial-skeletal syndrome [157]. Mutations in the gene CS-A, on chromosome 5, result in milder forms of the disease. For a recent review about clinical and genetic aspects of CS see Rapin et al [152]. Trichothiodystrophy (TTD) is a disease with a clinical phenotype overlapping that of XP and CS with microcephaly, mental retardation, ataxia, retinal and cochlear lesions, with the additional characteristic of sulfur-deficient brittle hair. TTD is associated with mutations in the genes XP-D, XP-B, and TTD-A (a TTD specific allele) [152,156]. Other autosomal recessive ataxia Other autosomal recessive ataxias include: cerebellar ataxia associated with deficiency in muscle coenzyme Q10 [158], infantile-onset SCA with sensory neuropathy [159] periodic vestibulocerebellar ataxia [160], ataxia and familial Gerhardt's syndrome [161], cerebellar ataxia and Whipple's disease [162], cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss (CAPOS) syndrome [163], posterior column ataxia and retinitis pigmentosa [164], Boucher-Neuhauser syndrome and ataxia with hypogonadism (Holmes) syndromes [165], and various ataxic syndromes associated with deafness [4]. These syndromes are all defined purely on a clinical basis, with no genetic markers or testing being presently available. X-LINKED ATAXIA These are very rare syndromes, and different entities have been described under this condition. The syndromes are of childhood or juvenile onset with limb ataxia, dysarthria plus in some cases dementia, mental retardation, and hearing impairment. Like pyruvate dehydrogenase complex defects (deficits in the E1a subunit of the enzyme) most X-linked ataxias have metabolic causes. For a list of the various syndromes described with X-linked ataxia see Evidente et al [4]. THE CONGENIAL AND METABOLIC ATAXIA A congenital developmental defect, or a metabolic cause, should be suspected in very early onset progressive or intermittent ataxia. Congenital ataxia patients have hypotonia, abnormal motor development, and early signs of cerebellar dysfunction. Head CT scans or MRI studies in these patients may reveal the underlying morphological defect, such as dysgenesis or agenesis of the cerebellar vermis, cerebellar hemispheres and/or part of the brainstem. Specific congenital syndromes with ataxia are mentioned in Table 2. Metabolic intermittent ataxias are expected to be caused mainly by disorders in the urea cycle (ornithine transcarbamylase and arginino succinate synthase), some aminoacidurias (maple syrup urine disease, isovaleric acidosis, 2-glutaric aciduria, and Hartnup disease), and disorders of pyruvate carboxylase, pyruvate dehydrogenase and mitochondrial diseases (Table 2). Other early onset
- 16. metabolic diseases with ataxia include metachromatic leukodystrophy, multiple sulfatase deficiency, adrenoleukodystrophy, sialidosis type I and ceroid lipofuscinosis. Behr's syndrome represents a heterogeneous group of diseases, characterized by optic atrophy beginning in early childhood associated with ataxia, spasticity, mental retardation, and sensory loss [166,167]. Marinesco-Sjogren syndrome is a chylomicron retention disease [134]. Some childhood progressive ataxias present an even more complex picture, as many of these metabolic disorders could be manifested after childhood, in the adolescence or adult life, and ataxia may not be a recognizable sign. Syndromes with childhood or juvenile onset which could include ataxia are as follows. (a) Kallman syndrome (hypogonadotrophic hypogonadism and anosmia with associated eye movement abnormalities and cerebellar ataxia) with an estimated incidence of 1:10,000 in males and 1:50,000 in females [168]. (b) Pelizaeus-Merzbacher syndrome (PMS) (neonatal tremor and shaking movements of the head, abnormal eye movements, and progression to cerebellar ataxia, dysarthria and intention tremor of the upper limbs). The disease progresses, during the second decade of the life, with increasing abnormal movements and mild dementia in PMS type I or classical form. The connatal form or PMS type II shows a more severe presentation detectable at birth. The gene responsible of PMS encodes for the myelin proteolipid protein, and maps to Xq21 [169]. PMS is allelic with a distinct clinical entity: X- linked spastic paraplegia type 2 (SPG2) [170]. Both diseases are part of the group of leukodystrophies. (c) Deficit in hexosaminidase A and hexosaminidase AB, clinically similar to FRDA, is a slowly progressive ataxia with retained reflexes, intention tremor, early dysarthria and psychiatric components, without sensory involvement [171,172]. (d) Niemann-Pick disease type C (hepatosplenomegaly, supranuclear ophthalmoplegia, ataxia, seizures and progressive dementia, with onset in early childhood) is an inherited autosomal recessive cholesterol storage disorder caused by impaired intracellular cholesterol trafficking [173]. Patients of this group C Niemann-Pick disease, like group D, have normal levels of sphingomyelinase activity. The general incidence of the disease has been estimated at about 1:100,000 live births, although a higher prevalence is recognized in isolated populations [173]. A major form of the disease (about 95% of the cases) is caused by mutations in the gene NPC1[174,175] on 18q11. The causative second gene, NPC2, has not been identified yet. (e) Krabbe's disease is an autosomal recessive disorder caused by galactocerebrosidase deficiency (EC 3.2.1.46), leading to the accumulation of galactosylceramide. Developmental delay, extreme irritability and crying followed by limb stiffness, are key signs. About 10% of the patients have a childhood onset with ataxia, dysarthria, limb paresthesias and weakness, visual loss, and a very variable rate of mental and physical deterioration, or a young-adult onset with optic nerve pallor, weakness, spasticity, and sensory- motor demyelinating neuropathy [176]. Patients with suspected metachromatic leukodystrophy or adrenoleukodystrophy could also have Krabbe's disease. (f) A deficit in galactose-1-phosphate-uridyl transferase, may lead to late neurological manifestation with cerebellar syndrome and abnormal movements as a consequence of galactosemia [177]. Peroxisomal disorders Peroxisomal disorders are autosomal recessive or X-linked metabolic diseases of neonatal or childhood onset, characterized by facial dysmorphism, hepatomegaly and complex neurological symptoms, including retinopathy, impaired hearing, hypotonia, psychomotor delay and seizures [178]. Infantile Refsum disease (RD) is a severe and slowly progressive peroxisomal disorder, where ataxia could be a predominant sign [179]. The characteristics of the syndrome are dysmorphic facial features, ataxia, retinitis pigmentosa, deafness, peripheral neuropathy, hepatomegaly, elevated protein levels in the CSF and the accumulation of phytanic acid in blood and tissues. RD is caused by phytanoyl-CoA hydroxylase deficiency [180–182]. Mitochondrial diseases A detailed description of mitochondrial diseases, which have variable multisystemic clinical features and complex genotype-phenotype correlations, is beyond the scope of this review. Another article in this issue describes these diseases in detail. Concerning ataxia, a mitochondrial basis for any ataxic patient should be suspected when ataxia is associated with or followed by a variable combination of progressive external ophthalmoplegia, myoclonic seizures, myopathy, cardiomyopathy, myoglobinuria, neuropathy, retinopathy, optic atrophy, hearing loss, and other systemic manifestations such as diabetes, renal tubular acidosis, and gastrointestinal disorders. The patient with sporadic ataxia The dramatic advances in our understanding of the molecular basis of genetic ataxia are diverting our attention from sporadic ataxias; however, many inherited ataxias can present without a family history, and the diagnosis of a genetic ataxia in such cases could be challenging. Conversely, patients with ataxia who have a family history may not always have hereditary ataxia. Ataxia may be due to a coincidental occurrence of a relatively common neurological
- 17. disease such as multiple sclerosis. Furthermore, the apparently positive family history may be based on ambiguous and inaccurate data. In these circumstances, differentiating hereditary ataxia from sporadic ataxia is an important diagnostic step. Although reviewing sporadic ataxias is beyond the scope of this review, a list of conditions to be considered for the differential diagnosis of sporadic ataxia are: (a) cerebral tumors; (b) paraneoplastic ataxia [183– 186]; (c) celiac disease [187,188] and Ramsay Hunt syndrome with CSF anti-gliadin antibodies [189]; (d) stroke; (e) hypothyroidism [190]; (f) toxins, metals, and drugs such as chronic exposures to alcohol, thallium, toluene, lithium ion, organic mercury, barbiturates, phenytoin, etc., (g) nutritional deficiencies (chronic alcoholism, celiac diseases and/or malabsorption syndromes) leading to deficit in vitamin B1, B12 and E, (h) multiple sclerosis, (i) infectious or post-infectious causes, and (j) neurodegenerative processes like multiple system atrophy (MSA) [191], and progressive supranuclear palsy (PSP) [192–197]. Even when ataxia is not a characteristic feature of some diseases, motor impairments other than ataxia may be presented with postural instability, oculomotor manifestations, and dysarthria in the early stages of these diseases. DIAGNOSTIC STRATEGY FOR ATAXIC SYNDROMES Determining the inheritance pattern by obtaining detailed family history is a critical step in establishing an accurate diagnosis of inherited ataxia. Unfortunately, this is often a neglected part of neurological evaluation. The family history must be detailed enough to differentiate autosomal, X-linked vs. mitochondrial inheritance, and dominant vs. recessive inheritance. Further information such as the penetrance, anticipation, and other phenotypic variations among affected members should be documented. Early parental death, adoption, non-paternity, ethnic background, and concomitant disorders within the family should be included in the history. A detailed clinical documentation of the ataxic syndrome, including the age of onset, the course of the disease, anatomical localization of the neurological deficits, and associated extra-nervous system symptoms and signs in the patient and the affected family members, also provides important clues for the correct diagnosis. Most early onset (childhood or second decade of life) nonmetabolic disorders are autosomal recessive while later onset (after 20y) suggests autosomal dominant disorders (although there are exceptions) (Table 2). Nonmetabolic hereditary ataxias generally show an insidious onset followed by a slowly progressive course. For recessive ataxias, differential diagnosis between treatable syndromes (Wilson disease, AVED, BKD and hypolipoproteinemia) and non-treatable ones (FRDA and most other recessive ataxias) at an early stage of the disease is critical because early treatment can prevent disease progression. For autosomal dominant ataxias, a first step is to recognize the ADCA clinical types (I, II or III). Although this approach is not always useful because of the variable overlaps between different dominant SCAs, ADCA subtypes may provide important diagnostic clues in many cases. Some additional clinical characteristics could help with the differential diagnosis, although there are, again, limitations in this approach (Table 1). Areflexia has been proposed as a main characteristic of SCA2; however, a SCA2 family has recently been found with brisk reflexes as a very early sign of the disease, which remains until advanced stages (A. L. Rosa, unpublished). Other useful diagnostic pearls include: (a) very early slow saccades in SCA2; (b) ataxia and blindness with marked anticipation, under-represented paternal transmissions in the pedigree in SCA7; (c) epilepsy in some members of a family with relatively pure cerebellar ataxia in SCA10; (d) epilepsy, complex movement disorders, mental deteriorations in the absence of macular degeneration in DRPLA and SCA17; (e) ADCA-I with conspicuous upper motor neuron signs in SCA1 and SCA 3; (f) and episodic ataxia in EA1, EA2, and an early stage of some SCA6 cases (see Table 1). The ethnic and/or geographic background may also provide some clues. SCA3/Machado-Joseph disease (MJD), originally described in two MJD families of Azorean descent, is now found in most ethnic populations around the world. SCA3 appears to be the most common SCA in many countries such as United States, China and Germany. SCA1 and SCA2 appear to be more common in the United Kingdom and Italy, and SCA2 in India and Cuba, while SCAs 3 and 6 appear to predominate in Japan. SCA10 has been exclusively seen in Mexicans. DRPLA is rare outside Japan. SCAs12, 13 and 14 have been reported in German American, French, and Japanese families, respectively. SCA17 may be relatively more frequent in Japan. FRDA has never been reported in sub-Saharan Africans, East Asians, and Native Americans. A founder effect could create geographically different prevalence within the same ethnic population. If clinical evaluation suggests a specific genetic disorder for which genetic testing is available, DNA testing should be considered as the first diagnostic test. A positive DNA test provides the most cost-effective and definitive diagnosis; however, if the DNA test is negative, it only excludes the disease tested. In some diseases caused by repeat expansions, there may be intermediate alleles whose diagnostic value is ambiguous. Some intermediate alleles may represent mutable normal alleles that cause no disease in the individual may expand to the full mutation range in the offspring. Other intermediate alleles may have reduced penetrance. Algorithms for DNA testing strategies for ataxic disorders have been discussed [97]. Presymptomatic and prenatal testing is available for many hereditary
- 18. ataxias through DNA analyses; however, ethical, legal and social ramifications of DNA testing are complex and require appropriate counseling by qualified professionals such as geneticists or genetic counselors. The importance of genetic counseling cannot be overemphasized in these cases. If genetic testing is not available but there are other diagnostic tests specific for the suspected disorder, they should be obtained. MRI and CT of the central nervous system, as well as electrophysiological, biochemical and metabolic studies may be useful for establishing the diagnosis of other disorders and excluding secondary causes. SUMMARY SUMMARY Clinical differentiation of various causes of progressive ataxia can be difficult [199]. Pathologic examination of patients with progressive ataxia enables identification of three broad categories of diseases according to the site of the gross and histologic abnormalities [1 , 2]. These three categories are the spinocerebellar forms, of which Friedreich’s ataxia is the most common; the corticocerebellar atrophies; and the cerebellar-brainstem atrophies. Histopathologic features of Friedreich’s ataxia include neuronal loss in the spinal ganglia and Clarke’s column and loss of myelinated fibers, with compensatory gliosis of the fasciculus gracilis and cuneatus in the posterior columns and of the spinocerebellar and pyramidal tracts in the lateral columns of the spinal cord [199 , 200]. Spinal cord thinning is reported almost invariably in pathologic descriptions of the disease [1 , 200]. MR imaging can show selective loss of bulk in the cerebellum and brainstem in corticocerebellar and cerebellar- brainstem atrophies [199 , 203, 203] hich are associated with brainstem and cerebellar white matter signal changes in olivopontocerebellar atrophy [204]. Atrophy of the upper portion of the cervical spinal cord has been emphasized in prior MR studies of patients with Friedreich’s ataxia [203, 205, 206], but intramedullary signal abnormalities were not mentioned. MR imaging could be used to depict the changes in the white matter tracts that are known to occur in the cervical spinal cord of patients with Friedreich’s ataxia. Detection of such changes could increase information provided by MR imaging in patients with Friedreich’s ataxia and be helpful for differential diagnosis in patients with progressive ataxia of uncertain clinical type. Loss of myelinated fibers and gliosis in the posterior and lateral columns of the spinal cord are histopathologic hallmarks of Friedreich’s ataxia. These are accompanied by atrophy of the upper portion of the spinal cord. MR imaging can be used to detect signal changes in the white matter tracts of the cervical spinal cord in patients with Friedreich’s ataxia. The anteroposterior diameter of the spinal cord is decreased in patients with Friedreich’s ataxia in the upper cervical spinal cord. Abnormal signal in the posterior or lateral columns of the spinal cord is observed on sagittal and/or axial images in patients with Friedreich’s ataxia and is not observed in other patients with corticocerebellar atrophies; and the cerebellar-brainstem atrophies. MR images of the cervical spinal cord in patients with Friedreich’s ataxia show thinning and intramedullary signal changes in the cervical portion of the spinal cord, consistent with degeneration of posterior and lateral white matter tracts. These MR findings might be helpful for differential diagnosis in patients with progressive ataxia of uncertain clinical type. Addendum A new version of this PDF file (with a new case) is uploaded in my web site every week (every Saturday and remains available till Friday.)
- 19. To download the current version follow the link "http://pdf.yassermetwally.com/case.pdf". You can also download the current version from my web site at "http://yassermetwally.com". To download the software version of the publication (crow.exe) follow the link: http://neurology.yassermetwally.com/crow.zip The case is also presented as a short case in PDF format, to download the short case follow the link: http://pdf.yassermetwally.com/short.pdf At the end of each year, all the publications are compiled on a single CD-ROM, please contact the author to know more details. Screen resolution is better set at 1024*768 pixel screen area for optimum display. Also to view a list of the previously published case records follow the following link (http://wordpress.com/tag/case-record/) or click on it if it appears as a link in your PDF reader To inspect the patient's full radiological study, click on the attachment icon (The paper clip icon in the left pane) of the acrobat reader then double click on the attached file. Click here to download the short case version of this case record in PDF format REFERENCES References [1]. Holmes GA. A form of familiar degeneration of the cerebellum. Brain. 1907;30:545-567 [2]. Greenfield JG. The spino-cerebellar degenerations. Oxford: Blackwell Scientific 1954 [3]. Harding A. Genetic aspects of autosomal dominant late onset cerebellar ataxia. J Med Genet. 1981;18(6):436- 441 [4]. Evidente V, Gwinn-Hardy K, Caviness J, et al. Hereditary ataxias. Mayo Clin Proc. 2000;75(5):475-490 [5]. Klockgether T, Wullner U, Spauschus A, et al. The molecular biology of the autosomal-dominant cerebellar ataxias. Mov Disord. 2000;15:604-612 [6]. Stevanin G, Durr A, Brice A. Clinical and molecular advances in autosomal dominant cerebellar ataxias: from genotype to phenotype and physiopathology. Eur J Hum Genet. 2000;8:4-18 [7]. Subramony S, Vig P, McDaniel D. Dominantly inherited ataxias. Semin Neurol. 1999;19(4):419-425 [8]. Harding A. Friedreich's ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 1981;104:589-620 [9]. Harding AE. Clinical features and classification of inherited ataxias. Adv Neurol. 1993;61:1-4 [10]. Orr HT. Beyond the Qs in the polyglutamine diseases. Genes Dev. 2001;15:925-932 [11]. Davies SW, Beardsall K, Turmaine M, et al. Are neuronal intranuclear inclusions the common neuropathology of triplet-repeat disorders with polyglutamine-repeat expansions?. Lancet. 1997;351:131-133 [12]. Perutz MF. Glutamine repeats and neurodegenerative diseases: molecular aspects. Trends Biochem Sci. 1999;24:58-63 [13]. Klement IA, Skinner PJ, Kaytor MD, et al. Ataxin-1 nuclear localization and aggregation: role in polyglutamine-induced disease in SCA1 transgenic mice. Cell. 1998;95:41-53 [14]. Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Ann Rev Neurosc. 2000;23:217-247 [15]. Yakura H, Wakisaka A, Fujimoto S, et al. Hereditary ataxia and HL-A. N Engl J Med. 1974;291(3):154-155 [16]. Banfi S, Servadio A, Chung M, et al. Identification and characterization of the gene causing type 1
- 20. spinocerebellar ataxia. Nat Genet. 1994;7:513-520 [17]. Sasaki H, Fukazawa T, Yanagihara T, et al. Clinical features and natural history of spinocerebellar ataxia type 1. Acta Neurol Scand. 1996;93:64-71 [18]. Genis D, Matilla T, Volpini V, et al. Clinical, neuropathologic, and genetic studies of a large spinocerebellar ataxia type 1 (SCA1) kindred: (CAG)n expansion and early premonitory signs and symptoms. Neurology. 1995;45:24-30 [19]. Ranum L, Chung M, Banfi S, et al. Molecular and clinical correlations in spinocerebellar ataxia type I: evidence for familial effects on the age at onset. Am J Hum Genet. 1994;55:244-252 [20]. Gispert S, Twells R, Orozco G, et al. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23–24.1. Nat Genet. 1993;4:295-299 [21]. Imbert G, Saudou F, Yvert G, et al. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. 1996;14:285-291 [22]. Pulst S, Nechiporuk A, Nechiporuk T, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14:269-276 [23]. Sanpei K, Takano H, Igarashi S, et al. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique. DIRECT. Nat Genet. 1996;14:277-284 [24]. Romanul F, Fowler H, Radvany J, et al. Azorean disease of the nervous system. N Engl J Med. 1977;296:1505- 1508 [25]. Kawaguchi Y, Okamoto T, Taniwaki M, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994;8:221-228 [26]. Cancel G, Abbas N, Stevanin G, et al. Marked phenotypic heterogeneity associated with expansion of a CAG repeat sequence at the spinocerebellar ataxia 3/Machado-Joseph disease locus. Am J Hum Genet. 1995;57:809-816 [27]. Matilla T, McCall A, Subramony S, et al. Molecular and clinical correlations in spinocerebellar ataxia type 3 and Machado-Joseph disease. Ann Neurol. 1995;38:68-72 [28]. Durr A, Stevanin G, Cancel G, et al. Spinocerebellar ataxia 3 and Machado-Joseph disease: clinical, molecular, and neuropathological features. Ann Neurol. 1996;39:490-499 [29]. Flanigan K, Gardner K, Alderson K, et al. Autosomal dominant spinocerebellar ataxia with sensory axonal neuropathy (SCA4): clinical description and genetic localization to chromosome 16q22.1. Am J Hum Genet. 1996;59:392-399 [30]. Nagaoka U, Takashima M, Ishikawa K, et al. A gene on SCA4 locus causes dominantly inherited pure cerebellar ataxia. Neurology. 2000;54:1971-1975 [31]. Takashima M, Ishikawa K, Nagaoka U, et al. A linkage disequilibrium at the candidate gene locus for 16q- linked autosomal dominant cerebellar ataxia type III in Japan. J Hum Genet. 2001;46:167-171 [32]. Ranum L, Schut L, Lundgren J, et al. Spinocerebellar ataxia type 5 in a family descended from the grandparents of President Lincoln maps to chromosome 11. Nat Genet. 1994;8:280-284 [33]. Stevanin G, Herman A, Brice A, et al. Clinical and MRI findings in spinocerebellar ataxia type 5. Neurology. 1999;53:1355-1357 [34]. Gomez C, Thompson R, Gammack J, et al. Spinocerebellar ataxia type 6: gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol. 1997;42:933-950 [35]. Sugawara M, Toyoshima I, Wada C, et al. Pontine atrophy in spinocerebellar ataxia type 6. Eur Neurol.
- 21. 2000;43:17-22 [36]. Geschwind D, Perlman S, Figueroa K, et al. Spinocerebellar ataxia type 6. Frequency of the mutation and genotype-phenotype correlations. Neurology. 1997;49:1247-1251 [37]. Jodice C, Mantuano E, Veneziano L, et al. Episodic ataxia type 2 (EA2) and spinocerebellar ataxia type 6 (SCA6) due to CAG repeat expansion in the CACNA1A gene on chromosome 19p. Hum Mol Genet. 1997;6:1973- 1978 [38]. Murata Y, Kawakami H, Yamaguchi S, et al. Characteristic magnetic resonance imaging findings in spinocerebellar ataxia 6. Arch Neurol. 1998;55:1348-1352 [39]. Nagai Y, Azuma T, Funauchi M, et al. Clinical and molecular genetic study in seven Japanese families with spinocerebellar ataxia type 6. J Neurol Sci. 1998;15:52-59 [40]. Zhuchenko O, Bailey J, Bonnen P, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62-69 [41]. Matsuyama Z, Kawakami H, Maruyama H, et al. Molecular features of the CAG repeats of spinocerebellar ataxia 6 (SCA6). Hum Mol Genet. 1997;6:1283-1287 [42]. Katayama T, Ogura Y, Aizawa H, et al. Nineteen CAG repeats of the SCA6 gene in a Japanese patient presenting with ataxia. J Neurol. 2000;247:711-712 [43]. Ishikawa K, Tanaka H, Saito M, et al. Japanese families with autosomal dominant pure cerebellar ataxia map to chromosome 19p13.1-p13.2 and are strongly associated with mild CAG expansions in the spinocerebellar ataxia type 6 gene in chromosome 19p13.1. Am J Hum Genet. 1997;61:336-346 [44]. Riess O, Schols L, Bottger H, et al. SCA6 is caused by moderate CAG expansion in the alpha1A-voltage- dependent calcium channel gene. Hum Mol Genet. 1997;6:1289-1293 [45]. Shimazaki H, Takiyama Y, Sakoe K, et al. Meiotic instability of the CAG repeats in the SCA6/CACNA1A gene in two Japanese SCA6 families. J Neurol Sci. 2001;185:101-107 [46]. Shizuka M, Watanabe M, Ikeda Y, et al. Molecular analysis of a de novo mutation for spinocerebellar ataxia type 6 and (CAG)n repeat units in normal elder controls. J Neurol Sci. 1998;161:85-87 [47]. Kato T, Tanaka F, Yamamoto M, et al. Sisters homozygous for the spinocerebellar ataxia type 6 (SCA6)/CACNA1A gene associated with different clinical phenotypes. Clin Genet. 2000;58:69-73 [48]. Ikeuchi T, Takano H, Koide R, et al. Spinocerebellar ataxia type 6: CAG repeat expansion in alpha1A voltage- dependent calcium channel gene and clinical variations in Japanese population. Ann Neurol. 1997;42:879-884 [49]. Matsuura T, Yamagata T, Burgess D, et al. Large expansion of the ATTCT pentanucleotide repeat in spinocerebellar ataxia type 10. Nat Genet. 2000;26:191-194 [50]. Takiyama Y, Sakoe K, Namekawa M, et al. A Japanese family with spinocerebellar ataxia type 6 which includes three individuals homozygous for an expanded CAG repeat in the SCA6/CACNL1A4 gene. J Neurol Sci. 1998;158:141-147 [51]. Stevanin G, Durr A, David G, et al. Clinical and molecular features of spinocerebellar ataxia type 6. Neurology. 1997;49:24-36 [52]. Ishikawa K, Fujigasaki H, Saegusa H, et al. Abundant expression and cytoplasmic aggregations of [alpha]1A voltage-dependent calcium channel protein associated with neurodegeneration in spinocerebellar ataxia type 6. Hum Mol Genet. 1999;8:1185-1193 [53]. Ishikawa K, Owada K, Ishida K, et al. Cytoplasmic and nuclear polyglutamine aggregates in SCA6 Purkinje cells. Neurology. 2001;56:1753-1756
- 22. [54]. Restituito S, Thompson R, Eliet J, et al. The polyglutamine expansion in spinocerebellar ataxia type 6 causes a beta subunit-specific enhanced activation of P/Q-type calcium channels in Xenopus oocytes. J Neurosci. 2000;20:6394-6403 [55]. Gomez C. Polyglutamine aggregates in SCA6 Purkinje cells: a tail of two toxicities. Neurology. 2001;56:1618- 1619 [56]. David G, Durr A, Stevanin G, et al. Molecular and clinical correlations in autosomal dominant cerebellar ataxia with progressive macular dystrophy (SCA7). Hum Mol Genet. 1998;7:165-167 [57]. Oh A, Jacobson K, Jen J, et al. Slowing of voluntary and involuntary saccades: an early sign in spinocerebellar ataxia type 7. Ann Neurol. 2001;49:801-804 [58]. Benton C, de Silva R, Rutledge S, et al. Molecular and clinical studies in SCA-7 define a broad clinical spectrum and the infantile phenotype. Neurology. 1998;51:1081-1086 [59]. David G, Abbas N, Stevanin G, et al. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat Genet. 1997;17:65-70 [60]. Koob M, Benzow K, Bird T, et al. Rapid cloning of expanded trinucleotide repeat sequences from genomic DNA. Nat Genet. 1998;18:72-75 [61]. Del-Favero J, Krols L, Michalik A, et al. Molecular genetic analysis of autosomal dominant cerebellar ataxia with retinal degeneration (ADCA type II) caused by CAG triplet repeat expansion. Hum Mol Genet. 1998;7:177-186 [62]. Johansson J, Forsgren L, Sandgren O, et al. Expanded CAG repeats in Swedish spinocerebellar ataxia type 7 (SCA7) patients: effect of CAG repeat length on the clinical manifestation. Hum Mol Genet. 1998;7:171-176 [63]. Giunti P, Stevanin G, Worth P, et al. Molecular and clinical study of 18 families with ADCA type II: evidence for genetic heterogeneity and de novo mutation. Am J Hum Genet. 1999;64:1594-1603 [64]. Stevanin G, Giunti P, Belal G, et al. De novo expansion of intermediate alleles in spinocerebellar ataxia 7. Hum Mol Genet. 1998;7:1809-1813 [65]. Gouw L, Castaneda M, McKenna C, et al. Analysis of the dynamic mutation in the SCA7 gene shows marked parental effects on CAG repeat transmission. Hum Mol Genet. 1998;7:525-532 [66]. Monckton D, Cayuela M, Gould F, et al. Very large (CAG)(n) DNA repeat expansions in the sperm of two spinocerebellar ataxia type 7 males. Hum Mol Genet. 1999;8:2473-2478 [67]. Cancel G, Duyckaerts C, Holmberg M, et al. Distribution of ataxin-7 in normal human brain and retina. Brain. 2000;123(Pt12):2519-2530 [68]. Einum D, Townsend J, Ptacek L, et al. Ataxin-7 expression analysis in controls and spinocerebellar ataxia type 7 patients. Neurogenetics. 2001;3:83-90 [69]. Kaytor M, Duvick L, Skinner P, et al. Nuclear localization of the spinocerebellar ataxia type 7 protein, ataxin- 7. Hum Mol Genet. 1999;8:1657-1664 [70]. Yvert G, Lindenberg KS, Picaud S, et al. Expanded polyglutamines induce neurodegeneration and transneuronal alterations in cerebellum and retina of SCA7 transgenic mice. Hum Mol Genet. 2000;9:2491-2506 [71]. Day J, Schut L, Moseley M, et al. Spinocerebellar ataxia type 8: clinical features in a large family. Neurology. 2000;55:649-657 [72]. Juvonen V, Hietala M, Paivarinta M, et al. Clinical and genetic findings in Finnish ataxia patients with the spinocerebellar ataxia 8 repeat expansion. Ann Neurol. 2000;48:354-361 [73]. Koob M, Moseley M, Schut L, et al. An untranslated CTG expansion causes a novel form of spinocerebellar
- 23. ataxia (SCA8). Nat Genet. 1999;21:379-384 [74]. Moseley M, Schut L, Bird T, et al. SCA8 CTG repeat: en masse contractions in sperm and intergenerational sequence changes may play a role in reduced penetrance. Hum Mol Genet. 2000;9:2125-2130 [75]. Silveira I, Alonso I, Guimaraes L, et al. High germinal instability of the (CTG)n at the SCA8 locus of both expanded and normal alleles. Am J Hum Genet. 2000;66:830-840 [76]. Ikeda Y, Shizuka-Ikeda M, Watanabe M, et al. Asymptomatic CTG expansion at the SCA8 locus is associated with cerebellar atrophy on MRI. J Neurol Sci. 2000;182:76-79 [77]. Nemes J, Benzow K, Moseley M, et al. The SCA8 transcript is an antisense RNA to a brain-specific transcript encoding a novel actin-binding protein (KLHL1). Hum Mol Genet. 2000;9:1543-1551 [78]. Worth PF, Houlden H, Giunti P, et al. Large, expanded repeats in SCA8 are not confined to patients with cerebellar ataxia. Nat Genet. 2000;24:214 [79]. Stevanin G, Herman A, Durr A, et al. Are (CTG)n expansions at the SCA8 locus rare polymorphisms?. Nat Genet. 2000;24:213-215 [80]. Vincent J, Neves-Pereira M, Paterson A, et al. An unstable trinucleotide-repeat region on chromosome 13 implicated in spinocerebellar ataxia: a common expansion locus. Am J Hum Genet. 2000;66:819-829 [81]. Rasmussen A, Matsuura T, Ruano L, et al. Clinical and genetic analysis of four Mexican families with spinocerebellar ataxia type 10. Ann Neurol. 2001;50:234-239 [82]. Worth PF, Giunti P, Gardner-Thorpe C, et al. Autosomal dominant cerebellar ataxia type III : linkage in a large British family to a 7.6-cM region on chromosome 15q14–21.3. Am J Hum Genet. 1999;65:420-426 [83]. O'Hearn E, Holmes S, Calvert P, et al. SCA-12: Tremor with cerebellar and cortical atrophy is associated with a CAG repeat expansion. Neurology. 2001;56:299-303 [84]. Holmes S, O'Hearn E, McInnis M, et al. Expansion of a novel CAG trinucleotide repeat in the 5' region of PPP2R2B is associated with SCA12. Nat Genet. 1999;23:391-392 [85]. Fujigasaki H, Verma I, Camuzat A, et al. SCA12 is a rare locus for autosomal dominant cerebellar ataxia: a study of an Indian family. Ann Neurol. 2001;49:117-121 [86]. Herman-Bert A, Stevanin G, Netter J, et al. Mapping of spinocerebellar ataxia 13 to chromosome 19q13.3- q13.4 in a family with autosomal dominant cerebellar ataxia and mental retardation. Am J Hum Genet. 2000;67:229-235 [87]. Yamashita I, Sasaki H, Yabe I, et al. A novel locus for dominant cerebellar ataxia (SCA14) maps to a 10.2-cM interval flanked by D19S206 and D19S605 on chromosome 19q13.4-qter. Ann Neurol. 2000;48:156-163 [88]. Miyoshi Y, Yamada T, Tanimura M, et al. A novel autosomal dominant spinocerebellar ataxia (SCA16) linked to chromosome 8q22.1–24.1. Neurology. 2001;57:96-100 [89]. Koide R, Kobayashi S, Shimohata T, et al. A neurological disease caused by an expanded CAG trinucleotide repeat in the TATA-binding protein gene: a new polyglutamine disease?. Hum Mol Genet. 1999;8:2047-2053 [90]. Zuhlke C, Hellenbroich Y, Dalski A, et al. Different types of repeat expansion in the TATA-binding protein gene are associated with a new form of inherited ataxia. Eur J Hum Genet. 2001;9:160-164 [91]. Nakamura K, Jeong S, Uchihara T, et al. SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein. Hum Mol Genet. 2001;10:1441-1448 [92]. Devos D, Schraen-Maschke S, Vuillaume I, et al. Clinical features and genetic analysis of a new form of spinocerebellar ataxia. Neurology. 2001;56:234-238
- 24. [93]. Potter N, Meyer M, Zimmerman A, et al. Molecular and clinical findings in a family with dentatorubral- pallidoluysian atrophy. Ann Neurol. 1995;37:273-277 [94]. Koide R, Ikeuchi T, Onodera O, et al. Unstable expansion of CAG repeat in hereditary dentatorubral- pallidoluysian atrophy (DRPLA). Nat Genet. 1994;6:9-13 [95]. Nagafuchi S, Yanagisawa H, Sato K, et al. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet. 1994;6:14-18 [96]. Nielsen J, Sorensen S, Hasholt L, et al. Dentatorubral-pallidoluysian atrophy. Clinical features of a five- generation Danish family. Mov Disord. 1996;11:533-541 [97]. Warner T, Williams L, Walker R, et al. A clinical and molecular genetic study of dentatorubropallidoluysian atrophy in four European families. Ann Neurol. 1995;37:452-459 [98]. Borke J, Wingfield M, Lewis K, et al. The Haw River Syndrome: dentatorubropallido-luysian atrophy (DRPLA) in an African-American family. Nat Genet. 1994;7:521-524 [99]. Browne D, Gancher S, Nutt J, et al. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136-140 [100]. Ophoff R, Terwindt G, Vergouwe M, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543-552 [101]. Tournier-Lasserve E. CACNA1A mutations: hemiplegic migraine, episodic ataxia type 2, and the others. Neurology. 1999;53:3-4 [102]. Denier C, Ducros A, Durr A, et al. Missense CACNA1A mutation causing episodic ataxia type 2. Arch Neurol. 2001;58:292-295 [103]. Jen J, Geschwind D. Ataxia and calcium channels: what a headache!. Arch Neurol. 2001;58:179-180 [104]. Griggs R, Moxley 3rd R, Lafrance R, et al. Hereditary paroxysmal ataxia: response to acetazolamide. Neurology. 1978;28:1259-1264 [105]. Battistini S, Stenirri S, Piatti M, et al. A new CACNA1A gene mutation in acetazolamide-responsive familial hemiplegic migraine and ataxia. Neurology. 1999;53:38-43 [106]. Auburger G, Ratzlaff T, Lunkes A, et al. A gene for autosomal dominant paroxysmal choreoathetosis/spasticity (CSE) maps to the vicinity of a potassium channel gene cluster on chromosome 1p, probably within 2 cM between D1S443 and D1S197. Genomics. 1996;31:90-94 [107]. Cossee M, Schmitt M, Campuzano V, et al. Evolution of the Friedreich's ataxia trinucleotide repeat expansion: founder effect and premutations. Proc Natl Acad Sci USA. 1997;94:7452-7457 [108]. Labuda M, Labuda D, Miranda C, et al. Unique origin and specific ethnic distribution of the Friedreich ataxia GAA expansion. Neurology. 2000;54:2322-2334 [109]. Harding A, Hewer R. The heart disease of Friedreich's ataxia: a clinical and electrocardiographic study of 115 patients, with an analysis of serial electrocardiographic changes in 30 cases. Q J Med. 1983;52:489-502 [110]. Finocchiaro G, Baio G, Micossi P, et al. Glucose metabolism alterations in Friedreich's ataxia. Neurology. 1988;38:1292-1296 [111]. Oppenheimer D. Brain lesions in Friedreich's ataxia. Can J Neurol Sci. 1979;6:173-176 [112]. Pandolfo M. Molecular basis of Friedreich ataxia. Mov Disord. 2001;16:815-821 [113]. Puccio H, Koenig M. Recent advances in the molecular pathogenesis of Friedreich ataxia. Hum Mol Genet.
- 25. 2000;9:887-892 [114]. Campuzano V, Montermini L, Molto M, et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423-1427 [115]. Forrest S, Knight M, Delatycki M, et al. The correlation of clinical phenotype in Friedreich ataxia with the site of point mutations in the FRDA gene. Neurogenetics. 1998;1:253-257 [116]. Klockgether T, Chamberlain S, Wullner U, et al. Late-onset Friedreich's ataxia. Molecular genetics, clinical neurophysiology, and magnetic resonance imaging. Arch Neurol. 1993;50:803-806 [117]. Ragno M, De Michele G, Cavalcanti F, et al. Broadened Friedreich's ataxia phenotype after gene cloning. Minimal GAA expansion causes late-onset spastic ataxia. Neurology. 1997;49:1617-1620 [118]. Christodoulou K, Deymeer F, Serdaroglu P, et al. Mapping of the second Friedreich's ataxia (FRDA2) locus to chromosome 9p23-p11: evidence for further locus heterogeneity. Neurogenetics. 2001;3:127-132 [119]. Babcock M, de Silva D, Oaks R, et al. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science. 1997;276:1709-1712 [120]. Foury F, Cazzalini O. Deletion of the yeast homologue of the human gene associated with Friedreich's ataxia elicits iron accumulation in mitochondria. FEBS Lett. 1997;411:373-377 [121]. Gibson T, Koonin E, Musco G, et al. Friedreich's ataxia protein: phylogenetic evidence for mitochondrial dysfunction. Trends Neurosci. 1996;19:465-468 [122]. Koutnikova H, Campuzano V, Foury F, et al. Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nat Genet. 1997;16:345-351 [123]. Rotig A, de Lonlay P, Chretien D, et al. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet. 1997;17:215-217 [124]. Wilson RB, Roof DM. Respiratory deficiency due to loss of mitochondrial DNA in yeast lacking the frataxin homologue. Nat Genet. 1997;16:352-357 [125]. Bradley J, Blake J, Chamberlain S, et al. Clinical, biochemical and molecular genetic correlations in Friedreich's ataxia. Hum Mol Genet. 2000;9:275-282 [126]. Rustin P, von Kleist-Retzow J, Chantrel-Groussard K, et al. Effect of idebenone on cardiomyopathy in Friedreich's ataxia: a preliminary study. Lancet. 1999;354:477-479 [127]. Puccio H, Simon D, Cossee M, et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet. 2001;27:181-186 [128]. Harding A. Early onset cerebellar ataxia with retained tendon reflexes: a clinical and genetic study of a disorder distinct from Friedreich's ataxia. J Neurol Neurosurg Psychiatry. 1981;44:503-508 [129]. Marzouki N, Belal S, Benhamida C, et al. Genetic analysis of early onset cerebellar ataxia with retained tendon reflexes in four Tunisian families. Clin Genet. 2001;59:257-262 [130]. Palau F, De Michele G, Vilchez J, et al. Early-onset ataxia with cardiomyopathy and retained tendon reflexes maps to the Friedreich's ataxia locus on chromosome 9q. Ann Neurol. 1995;37:359-362 [131]. Ouahchi K, Arita M, Kayden H, et al. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat Genet. 1995;9:141-145 [132]. Catignani G, Bieri J. Rat liver alpha-tocopherol binding protein. Biochim Biophys Acta. 1977;497:349-357 [133]. Cavalier L, Ouahchi K, Kayden H, et al. Ataxia with isolated vitamin E deficiency: heterogeneity of mutations
