Overview of Oligonucleotide Therapeutics & Its Delivery.pdf
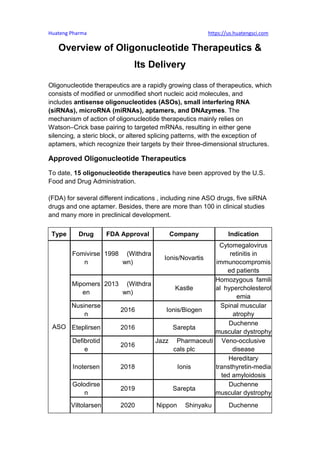
- 1. Huateng Pharma https://us.huatengsci.com Overview of Oligonucleotide Therapeutics & Its Delivery Oligonucleotide therapeutics are a rapidly growing class of therapeutics, which consists of modified or unmodified short nucleic acid molecules, and includes antisense oligonucleotides (ASOs), small interfering RNA (siRNAs), microRNA (miRNAs), aptamers, and DNAzymes. The mechanism of action of oligonucleotide therapeutics mainly relies on Watson–Crick base pairing to targeted mRNAs, resulting in either gene silencing, a steric block, or altered splicing patterns, with the exception of aptamers, which recognize their targets by their three-dimensional structures. Approved Oligonucleotide Therapeutics To date, 15 oligonucleotide therapeutics have been approved by the U.S. Food and Drug Administration. (FDA) for several different indications , including nine ASO drugs, five siRNA drugs and one aptamer. Besides, there are more than 100 in clinical studies and many more in preclinical development. Type Drug FDA Approval Company Indication ASO Fomivirse n 1998 (Withdra wn) Ionis/Novartis Cytomegalovirus retinitis in immunocompromis ed patients Mipomers en 2013 (Withdra wn) Kastle Homozygous famili al hypercholesterol emia Nusinerse n 2016 Ionis/Biogen Spinal muscular atrophy Eteplirsen 2016 Sarepta Duchenne muscular dystrophy Defibrotid e 2016 Jazz Pharmaceuti cals plc Veno-occlusive disease Inotersen 2018 Ionis Hereditary transthyretin-media ted amyloidosis Golodirse n 2019 Sarepta Duchenne muscular dystrophy Viltolarsen 2020 Nippon Shinyaku Duchenne
- 2. Huateng Pharma https://us.huatengsci.com muscular dystrophy Casimerse n 2021 Sarepta Duchenne muscular dystrophy siRNA Patisiran 2018 Alnylam Hereditary transthyretin-media ted amyloidosis Givosiran 2019 Alnylam Acute hepatic porphyria Lumasiran 2020 Alnylam Primary hyperoxaluria type 1 Inclisiran 2021 Novartis Primary hyperchol esterolemia Vutrisiran 2022 Alnylam Hereditary transthyretin-media ted amyloidosis Aptam er Pegaptani b 2004 (Withdra wn) Pfizer/Eyetech Neovascular (Wet) Age-Related Macular Degeneration Table. Approved Oligonucleotide therapeutics It has been 25 years since fomivirsen, the first small oligonucleotide therapeutic, was introduced in 1998. However, the market for oligonucleotide therapeutics is still immature, and there is still a lot of room for development. In the past 5 years, the market for oligonucleotide therapeutics has maintained a promising growth momentum, with the market volume expanding from $2 billion in 2018 to nearly $4 billion today. Mechanism of Action of Oligonucleotide Drugs RNA interference (RNAi) RNAi is a process whereby long double-stranded RNA is sheared into short double-stranded RNA and then bound to a protein to form an RNA-induced silencing complex (RISC). After the degradation of the sense strand of the short-stranded RNA, RISC then binds to specific mRNAs to degrade the mRNAs and ultimately silence the expression of the corresponding genes. This is a highly conserved process.
- 3. Huateng Pharma https://us.huatengsci.com Figure. Mechanism of Action of RNAi [1] Antisense Oligonucleotides (ASO) ASO is a single-stranded DNA or RNA sequence complementary to the mRNA of a target gene, usually consisting of a dozen to several dozen bases, produced by chemical synthesis. After certain specific chemical modifications of ASO, ASO drugs enter the cell by certain means and are able to specifically regulate the expression of target genes. ASO targets various types of nucleic acids (pre-mRNA, mRNA, non-coding RNA) in the cell. ASO inhibits protein production primarily by stimulating RNAase H activity, which in turn leads to the degradation of the target mRNA (ASO "Gapmers"). Figure. Mechanism of Action of ASO [1] Aptamers
- 4. Huateng Pharma https://us.huatengsci.com Aptamers are single-stranded DNA or RNA molecules screened by large oligonucleotide libraries (called SELEX) that bind specific targets with high selectivity and specificity. Common targets include small metal ions and organic molecules, proteins, viruses, bacteria, and whole cells. Target recognition and binding involves three-dimensional, shape-dependent interactions as well as hydrophobic interactions. The diagram below shows aptamer Pegaptanib inhibiting the action of target protein VEGF-165 by binding to its receptor VEGFR. Figure. Mechanism of action of aptamers [1] Advantages of Oligonucleotide Drugs ❖ High specificity: Oligonucleotide drugs are artificially designed based on target RNA, so the target is clear and the target specificity is strong. ❖ Easy design and short R&D cycle: Preclinical development of oligonucleotide drugs is firstly done by determining gene sequences and making proper designs for disease genes to silence gene targets, so it can avoid blind development and save R&D time to a great extent. ❖ Rich targets: Oligonucleotide drugs are treated from post-transcriptional level, which can make a breakthrough for some special targets where protein targets can be efficacious, and are expected to overcome genetic diseases for which there is no drug yet.
- 5. Huateng Pharma https://us.huatengsci.com Figure. Oligonucleotide Drugs vs. small molecule drugs [2] Delivery of Oligonucleotide Drugs The key to the drug production of oligonucleotide drugs lies in chemical modification and delivery system technology. Unmodified Oligonucleotide Drugs are not only susceptible to degradation by nucleases in vivo, but also susceptible to induce immune reactions; moreover, without the help of targeted delivery systems, negatively charged oligonucleotide drugs are difficult to enter cells for action. Currently, the following delivery platform technologies are commonly used for oligonucleotide therapy. ❖ Antisense Oligonucleotides (ASOs) ❖ GalNAc-siRNA conjugates ❖ Lipid Nanoparticles (LNPs) ❖ Adeno-Associated Viral Vectors (AAV vectors) ❖ Exosomes
- 6. Huateng Pharma https://us.huatengsci.com Among them, the development of delivery carriers is more critical. At present, GalNAc-siRNA conjugates and LNPs are the most studied and discussed delivery systems in RNAi therapy owing to their practicality, stability, and safety. GalNac Conjugates GalNAc conjugation is emerging as a dominant strategy for delivery of therapeutic oligonucleotides. To date, four GalNAc-siRNA therapeutics, Leqvio® (inclisiran), GIVLAARI™ (givosiran), Oxlumo™ (lumasiran) and Amvuttra (vutrisiran), have been approved for commercial applications. Figure. Delivery of GalNAc-siRNA conjugates into hepatocytes. [3] GalNAc is a sugar molecule that can recognize and bind to a cell surface protein, the asialoglycoprotein receptor (ASGPR), which is abundantly expressed on liver cells (hepatocytes). GalNac-siRNA conjugates can rapidly enter the liver through the circulatory system after subcutaneous injection. It is then rapidly endocytosed by liver cells mediated by ASPGR and accumulated in lysosomes, slowly released and persistently loaded onto RISC, thus achieving long-acting inhibitory effects. At present, Alnylam has more comprehensive patent coverage on GalNac, covering GalNac-siRNA, including double-stranded nucleic acid structure, limiting some site-specific modifications, etc. Dicema mainly avoids patents by constructing single-stranded structures and end-loop formations, etc. In addition to Alnylam, GalNAc-based technology platforms have emerged in recent years, including Dicerna's GalXC, Arrowhead's TRiM, Ionis' LICA, etc. Lipid Nanoparticle (LNP)
- 7. Huateng Pharma https://us.huatengsci.com Lipid nanoparticles (LNPs) are chemically synthesized multicomponent lipid formulations (~100 nm in size) encapsulating siRNAs for delivery to the target tissue. Since RNA is negatively charged, positively charged lipids can bind to it, forming very small particles with a reverse interlaced structure, which are wrapped by phospholipids. LNPs typically consist of four components—ionizable lipid, phospholipid, cholesterol, and PEGylated lipid. Among them, PEGylated lipid can improve the hydrophilicity of the drug, avoid the rapid clearance of the drug by the immune system, prevent the aggregation of particles, and increase the stability. Huateng Pharma, as a leading supplier of PEG derivatives, can provide a wide range of PEG lipids to our clients worldwide. Conclusion Although the development of oligonucleotide therapies is still difficult, it is believed that with the continuous development, improvement and progress of related technologies, oligonucleotide therapies will surely set off a new wave in the pharmaceutical industry. References: [1] Ageliki Laina, et al. RNA Therapeutics in Cardiovascular Precision Medicine. Front Physiol. 2018 Jul 25;9:953. doi: 10.3389/fphys.2018.00953. eCollection 2018. [2] Phuc Tran, et al. Delivery of Oligonucleotides: Efficiency with Lipid Conjugation and Clinical Outcome. Pharmaceutics. 2022 Feb 1;14(2):342. doi: 10.3390/pharmaceutics14020342. [3]Aaron D. Springer and Steven F. Dowdy.GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics.Nucleic Acid Therapeutics.Jun 2018.109-118.http://doi.org/10.1089/nat.2018.0736