Carbon nanotubes
- 1. CARBON NANOTUBES PRESENTED BY :- JEMIN MANGUKIYA (19BME045)
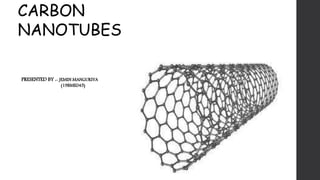
- 2. WHAT ARE CNTs ? • CARBON NANOTUBES (CNTS) ARE CYLINDRICAL MOLECULES THAT CONSIST OF ROLLED-UP SHEETS OF SINGLE-LAYER CARBON ATOMS • CARBON NANOTUBES (CNTS) ARE AN ALLOTROPE OF CARBON. • THEY ARE CHEMICALLY BONDED WITH SP2 BONDS, AN EXTREMELY STRONG FORM OF MOLECULAR INTERACTION. • NANOTUBES ARE MEMBERS OF THE FULLERENE STRUCTURAL FAMILY, WHICH ALSO INCLUDES BUCKYBALLS. • WHEREAS BUCKYBALLS ARE SPHERICAL IN SHAPE, A NANOTUBE IS CYLINDRICAL, WITH AT LEAST ONE END TYPICALLY CAPPED WITH A HEMISPHERE OF THE BUCKYBALL STRUCTURE
- 3. • A CARBON NANOTUBE HAS A HONEYCOMB LATTICE ROLLED INTO CYLINDER . • DEPENDING UPON THE STRUCTURE THERE ARE TWO TYPES OF CNTs. A FEW CONCENTRIC CYLINDERS WITH THE REGULAR PERIODIC INTERLAYER SPACING LOCATE AROUND ORDINARY CENTRAL HOLLOW AND MADE MWCNTs (MULTI WALLED CARBON NANOTUBES). • DEPENDING ON THE NUMBER OF LAYERS, THE INNER DIAMETER OF MWCNTs DIVERGES FROM 0.4 NM UP TO A FEW NANOMETERS AND OUTER DIAMETER VARIES CHARACTERISTICALLY FROM 2 NM UP TO 20 TO 30 NM. • MOREOVER THE ENDS ARE CAPPED WITH DOME SHAPED PENTAGONAL RINGS. ON OTHER HAND, SWCNT (SINGLE WALLED CARBON NANOTUBES) DIAMETERS DIFFER FROM 0.4 TO 2 TO 3 NM, AND THEIR LENGTH IS TYPICALLY OF THE MICROMETER RANGE. • SWCNTS USUALLY CAN COME TOGETHER AND FORM BUNDLES (ROPES). IN A BUNDLE STRUCTURE, SWCNTS ARE HEXAGONALLY ORGANIZED TO FORM A CRYSTAL-LIKE CONSTRUCTION STRUCTURE
- 4. FIGURE :- 1. SINGLE WALL CNT 2. MULTIWALL CNT
- 6. • THERE ARE SEVERAL TECHNIQUES THAT HAVE BEEN DEVELOPED FOR FABRICATING CNT STRUCTURES WHICH MAINLY INVOLVE GAS PHASE PROCESSES. COMMONLY, THREE PROCEDURES ARE BEING USED FOR PRODUCING CNTS: (1) THE CHEMICAL VAPOR DEPOSITION (CVD) TECHNIQUE (2) THE LASER-ABLATION TECHNIQUE , AND (3) THE CARBON ARC-DISCHARGE TECHNIQUE . (4) HIGH PRESSURE CARBON MONOXIDE DISPROPORTIONATION(HIPCO) • HIGH TEMPERATURE PREPARATION TECHNIQUES FOR EXAMPLE LASER ABLATION OR ARC DISCHARGE WERE FIRST USED TO SYNTHESIZE CNTS, BUT CURRENTLY, THESE TECHNIQUES HAVE BEEN SUBSTITUTED BY LOW TEMPERATURE CHEMICAL VAPOR DEPOSITION (CVD) METHODS (<800°C), SINCE THE NANOTUBE LENGTH, DIAMETER, ALIGNMENT, PURITY, DENSITY, AND ORIENTATION OF CNTS CAN BE ACCURATELY CONTROLLED IN THE LOW TEMPERATURE CHEMICAL VAPOR DEPOSITION (CVD) METHODS . SYNTHESIS
- 7. PROPERTIES 1. THEIR MECHANICAL TENSILE STRENGTH CAN BE 400 TIMES THAT OF STEEL . THEY ARE VERY LIGHT-WEIGHT – THEIR DENSITY IS ONE SIXTH OF THAT OF STEEL BECAUSE CARBON NANOTUBES HAVE THE SP2 BONDS BETWEEN THE INDIVIDUAL CARBON ATOMS AND THERE TENDENCY TO FORM ROPE TOGETHER VIA VANDER WAAL FORCE MAKES IT STRONGER AND LIGHT IN WEIGHT .EVEN STRONGER THAN SP3 BOND OF DAIMOND. 2. CNTs THERMAL CONDUCTIVITY IS BETTER THAN THAT OF A DIAMOND SINCE CARBON NANOTUBES HAVE SUCH A PERFET STRUCTURE , THEY AVOID DEGRADATION OF STRENGTH THAT YOU GET WITH OTHER MATERIAL THE STIFFNESS OF CARBON BOND HELPS TRANSMIT THIS VIBRATION THROUGHTOUT THE NANOTUBE PROVIDING GOOD THERMAL CONDUCTIVITY. 3. CARBON NANOTUBES HAVE A VERY HIGH ASPECT RATIO OF GREATER THAN 1000. IN OTHER WORDS, IN RELATION TO THEIR LENGTH, THEY ARE EXTREMELY THIN
- 8. 4. LIKE GRAPHITE, THEY ARE HIGHLY CHEMICALLY STABLE AND RESIST VIRTUALLY ANY CHEMICAL IMPACT UNLESS THEY ARE SIMULTANEOUSLY EXPOSED TO HIGH TEMPERATURES AND OXYGEN - A PROPERTY THAT MAKES THEM EXTREMELY RESISTANT TO CORROSION. 5. THEIR HOLLOW INTERIOR CAN BE FILLED WITH VARIOUS NANOMATERIALS, SEPARATING AND SHIELDING THEM FROM THE SURROUNDING ENVIRONMENT - A PROPERTY THAT IS EXTREMELY USEFUL FORNANOMEDICINE APPLICATIONS LIKE DRUG DELIVERY. 6. THE WAY OUTER WALL OF CARBON NANOTUBES ARE WRAPPED , MAKES IT CONDUCTIVE. THE WALL ATOMS ARE STRUCTURED IN A WAY THAT MAKES IT ROOM TEMPERATURE ZERO GAP SEMI CONDUCTIVE BY MAKING ELECTRONS FREE TO MOVE.
- 9. APPLICATIONS CNTS HAVE BEEN MAKING QUITE AN IMPRESSION ON COMMERCIAL PRODUCTS FOR SOME TIME NOW. CARBON NANOTUBES ARE ALREADY BEING USED TO CONTROL OR ENHANCE CONDUCTIVITY IN POLYMERS AND ARE ADDED TO ANTI-STATIC PACKAGING. THE MOST POPULAR CURRENT USE FOR CNTS IS STRUCTURAL REINFORCEMENT. THEY ARE ADDED TO OTHER MATERIALS LIKE REBAR TO CONCRETE BECAUSE OF THEIR HIGH STRENGTH, LOW WEIGHT, AND FLEXIBILITY. CNT PRODUCTION IS ALSO USED IN BULK COMPOSITE MATERIALS AND THIN FILMS. THESE ARE THE FEW PLACE FOR APPLICATION OF CNTs:- • CNTs field emission CNTs thermal conductivity • CNTs energy storage CNTs conductive properties • Molecular electronics based on CNTs CNTs structural applications • CNTs fibre and fabric CNTs biomedical applications • CNTs Air & Water Filtration CNTs catalyst supports
- 10. CNTS THERMAL CONDUCTIVITY AND EXPANSION CNTS HAVE OUTSTANDING HEAT CONDUCTIVITY, ELECTRICAL CONDUCTIVITY, AND MECHANICAL PROPERTIES. THEY ARE PROBABLY THE BEST ELECTRON FIELD-EMITTER POSSIBLE. CNTS ARE MOLECULARLY PERFECT, IN THE SENSE THAT THEY ARE GENERALLY FREE OF PROPERTY-DEGRADING FLAWS IN THE NANOTUBE STRUCTURE. THEIR MATERIAL PROPERTIES CAN THUS REACH CLOSE TO THE VERY HIGH LEVELS INTRINSIC TO THEM. DUE TO THESE EXTRAORDINARY CHARACTERISTICS, CNTS CAN BE PROSPECTIVELY USED IN A NUMBER OF APPLICATIONS. CNTS BIOMEDICAL APPLICATIONS SINCE A GREAT PART OF THE HUMAN BODY IS MADE UP OF CARBON, IT IS USUALLY CONSIDERED A VERY BIOCOMPATIBLE MATERIAL. THE GROWTH OF CELLS ON CNTS HAS BEEN DEMONSTRATED; THEREFORE, THEY APPARENTLY HAVE NO TOXIC EFFECT. THE ABILITY TO FUNCTIONALIZE (CHEMICALLY MODIFY) THE SIDEWALLS OF CNTS ALSO GIVES RISE TO BIOMEDICAL APPLICATIONS INCLUDING NEURON GROWTH AND REGENERATION, AND VASCULAR STENTS. IT HAS ALSO BEEN DEMONSTRATED THAT A SINGLE STRAND OF DNA CAN BE BONDED TO A NANOTUBE, WHICH CAN SUBSEQUENTLY BE EFFECTIVELY INSERTED INTO A CELL. CNTS AIR AND WATER FILTRATION WITH THE ABILTY TO BLOCK TINIEST PARTICLE AND DESTROING SMALLEST BATERIAL CNTS MAKES ITS USE IN PURIFICATION INDUSTRIES.
- 11. WASTE WATER TREATMENT CNTs HAS LARGE SURFACE AREA THAT GIVES LARGE CAPACITY TO RETAIN WATER SOLUBLE DRUG. SOLAR CELL DUE TO STRONG UV ABSORPTION CHARACTERSTICS , CNTs ARE POTENTIAL CANDIDATES TO BE USED FOR SOLAR CELL RESEARCH HAS SHOWN THAT THEY PROVIDE SIZABLE INCREASE IN EFFICIENCY, EVEN AT CURRENT UNOPTIMIZED STATE. HYDROGEN STORAGE BY TAKING ADVANTAGE OF CAPILLARY EFFECTS OF SMALL CARBON NANOTUBES, IT IS POSSIBLE TO CONDENSE GASES IN HIGH DENSITY INSIDE SINGLE WALL NANOTUBE. THIS ALLOWS FOR GASES,MOST NOTABLE HYDROGEN TO BE STORED AT HIGH DENSITIES WITHOUT BEING CONDENSED INTO LIQUID STATE. THESE METHOD CAN BE USED IN VEHICLES IN PLACE OF FUEL TANKS.