Apoptosis - An Introduction
- 1. An Introduction to Apoptosis By P B Mallikharjuna PhD GFGC, Yelahanka, Bangalore 15/06/2021
- 2. INTRODUCTION The formation of new cells by cell division and in turn the destruction of old cells by cell death go hand in hand in all multicellular organisms. Timely death & discard of old /unwanted /damaged cells is as important as creation of new cells.(Homeostasis) Programmed cell death (PCD) is a physiological cell death process involved in the selective elimination of unwanted cells. (Ellis et al.,1991) PCD occurs via apoptosis - a Greek word meaning “falling leaves” – coined by John Kerr et al.,(1972) “Apo- apart ; Ptosis- to drop/ to fall (Shedding of leaves from trees) Apoptosis is a form of death that the cell itself initiates, regulates, and executes using an elaborate arsenal of cellular and molecular machinery.
- 3. This phenomenon came into limelight in 1992 by the understanding the molecular basis of Apoptosis in Caenorahbditis elegans, a nematode by Brenner et al (received the 2002 Nobel Prize in Medicine) Apoptosis, or programmed cell death, is a highly regulated process that allows a cell to self-degrade in order for the body to eliminate unwanted or dysfunctional cells : genetically determined elimination of cells. During apoptosis, the genome of the cell will fracture, the cell will shrink and part of the cell will disintegrate into smaller apoptotic bodies. 50 to 70 billion cells die each day due to apoptosis in the average human adult. For an average child between the ages of 8 and 14, approximately 20 billion to 30 billion cells die a day
- 4. Why Cells Commit Suicide? • There seem to be two major reasons. First, apoptosis is one means by which a developing organism shapes its tissues and organs. a human fetus has webbed hands and feet early on its development. Later, apoptosis removes skin cells, revealing individual fingers and toes. A fetus’s eyelids form an opening by the process of apoptosis. During metamorphosis, tadpoles lose their tails through apoptosis. In young children, apoptosis is involved in the processes that literally shape the connections between brain cells, in mature females, apoptosis of cells in the uterus causes the uterine lining to slough off at each menstrual cycle.
- 5. Secondly ; Cells may also commit suicide in times of distress, for the good of the organism as a whole. For example, in the case of a viral infection, certain cells of the immune system, called cytotoxic T lymphocytes, bind to infected cells and trigger them to undergo apoptosis. Also, cells that have suffered damage to their DNA, which can make them prone to becoming cancerous, are induced to commit apoptosis.
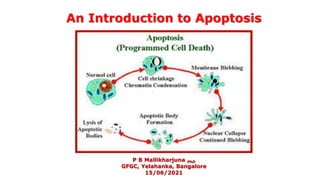
- 6. APOPTOSIS IN ANIMALS The process of apoptosis was well studied in animal cells which undergo orderly series of events both in the nucleus and in the cytoplasm. The cells shrink and develop bubble-like protrusions (technical name: “blebs”) on their surface. The DNA in the nucleus gets chopped up into small pieces, and some organelles of the cell, such as the endoplasmic reticulum, break down into fragments. In the end, the entire cell splits up into small chunks or apoptotic bodies , each neatly enclosed in a package of membrane. These apoptotic bodies release signals that attract debris-eating (phagocytic) immune cells, such as macrophages (T cells).
- 7. Also, the fragments of the dying cell display a lipid molecule called phosphatidylserine on their surface. Phosphatidylserine is usually hidden on the inside of the membrane, and when it is on the outside, it lets the phagocytes bind and "eat" the cell fragments
- 8. Apoptosis is mediated by proteolytic enzymes called caspases, which are synthesized in the precursor form as procaspases. When activated by various signals, caspases function to cause cell death in most organisms, ranging from C. elegans to human beings. Caspases are a family of cysteine proteases that cleave at specific aspartate‐containing sites. They participate in both the initiation of apoptosis and the disassembly of cellular contents More than 10 caspases have been identified. Some of them (e.g., caspase 8 and 10) are involved in the initiation of apoptosis, others (caspase 3, 6, and 7) execute the death order by destroying essential proteins in the cell.
- 9. The process of apoptosis is highly complex and sophisticated, involving an energy- dependent series of molecular events. Three different pathways work on different mechanisms to achieve apoptosis. All three pathways converge at the same terminal pathway, which results in the sequential degradation of cellular organelles. 1. Extrinsic or death receptor pathway : initiates apoptosis involves transmembrane receptor-mediated interactions. These interactions take place between ligands and their corresponding death receptors that are all part of the tumor necrosis factor (TNF) family. 2. The intrinsic or mitochondrial pathway: initiates apoptosis involves a series of non-receptor-mediated processes that produce intracellular signals and act directly on targets within the cell. This pathway involves mitochondrial-initiated events. The factors that initiate the intrinsic pathway produce intracellular signals that might act in either a positive or negative fashion.
- 10. 3. Perforin/granzyme pathway: is a novel pathway employed by cytotoxic T lymphocytes that exert their cytotoxic effects on tumor cells and virus-infected cells. This involves secretion of the transmembrane pore-forming molecule, perforin, with a subsequent release of cytoplasmic granules through the pore and towards the target cell.The granules consist of two crucial serine proteases; granzyme A and granzyme B that activate different proteins in the pathway. 4. Execution pathway: Both the extrinsic and intrinsic pathways end at the point of the execution phase, considered the terminal pathway of apoptosis.This phase of apoptosis is initiated by the activation of various caspases that activate cytoplasmic endonucleases and proteases. The cytoplasmic endonucleases degrade the nuclear material, whereas the proteases degrade the nuclear and cytoskeletal proteins.Caspase-3 is the most important protein of the executioner caspases and is activated by any of the initiator caspases (caspase-8, caspase-9, or caspase-10).
- 11. APOPTOSIS IN PLANTS Programmed cell death (PCD) occurs in many plant cells and tissues and is involved in numerous developmental and adaptive processes, including gamete formation; embryo development; degeneration of tissues in the seed and fruit ; tissue and organ development (4 through 6); senescence (7); and responses to environmental signals and pathogens
- 12. Types of PCD in plants
- 13. The general term for the dissolution of cytoplasm within the cell wall through the action of the cell’s own catabolic machinery is autolysis. plants employ different kinds of autolytic process during development and adaptation. One way that some plant cells can dispose of their cellular contents is through autophagy, death process in which the cell “eats itself ” from within. Vesicles are produced that engulf portions of the cytosol, including intact organelles. These vesicles, called autophagosomes, are taken up by the central vacuole of the cell ,or in some cases fuse with lysosomes (lytic vesicles), and are broken down by hydrolases.
- 14. Mechanistically, autophagy involves distinctive stages: (i) vesicle induction; (ii) vesicle expansion; (iii) tonoplast docking and fusion; and (iv) digestion. Molecular components regulating autophagy have been particularly well described in yeast, but homologs of most of these autophagic genes (ATG) are also present in Arabidopsis and other plant species. Tracheid development from a parenchyma cell due to Apoptosis
- 15. Initiation of autophagy is regulated by a target of rapamycin (TOR) kinase. Under normal conditions TOR kinase phosphorylates ATG1 kinase and accessory proteins, leading to their inactivation. To initiate autophagy, TOR kinase is inactivated, allowing ATG1 to interact with several ATG proteins, leading ultimately to the formation of a vacuolar‐sorting complex that includes a phosphatidylinositol 3‐kinase (PI3‐K) protein. This is a key vesicle nucleation step in which tubular pre‐autophagic structures (PASs) are formed, possibly from the endoplasmic reticulum (ER). PASs coalesce to form a cage that captures a portion of the cytoplasm.
