OHRP & the Requirement for IRB Review of Registries
•
1 j'aime•448 vues
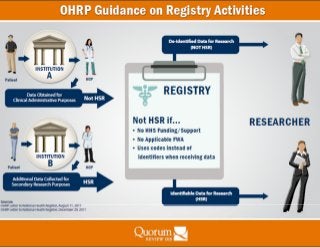
The Office for Human Research Protections (OHRP) recently posted correspondence with the National Health Registry in two letters dated August 11, 2011 and December 29, 2011 in hopes that the responses would be useful to sponsors, institutions, and IRBs. The correspondence relates primarily to the issue of whether various activities related to the registry require IRB review. The links below provide the full text of the letters: - See more at: http://www.quorumreview.com/ohrp-irb-review-registries/#sthash.UpYuS69f.dpuf
Signaler
Partager
Signaler
Partager
Télécharger pour lire hors ligne
Recommandé
Webinar: Transforming Operational Throughput – The Journey Toward Value-Based...

Webinar: Transforming Operational Throughput – The Journey Toward Value-Based...Huron Consulting Group
Pathways and targets how might these affect my treatment decisions gail eckh...

Pathways and targets how might these affect my treatment decisions gail eckh...Fight Colorectal Cancer
Recommandé
Webinar: Transforming Operational Throughput – The Journey Toward Value-Based...

Webinar: Transforming Operational Throughput – The Journey Toward Value-Based...Huron Consulting Group
Pathways and targets how might these affect my treatment decisions gail eckh...

Pathways and targets how might these affect my treatment decisions gail eckh...Fight Colorectal Cancer
Webinar Slides: Biobanking & Future Research: Addressing the "Unknown" in the...

Webinar Slides: Biobanking & Future Research: Addressing the "Unknown" in the...Quorum Review - Independent Review Board
Webinar: Research Involving Subjects with Limited Capacity: IRB Expectations ...

Webinar: Research Involving Subjects with Limited Capacity: IRB Expectations ...Quorum Review - Independent Review Board
E consent for research: Considerations in Implementation and IRB Review

E consent for research: Considerations in Implementation and IRB ReviewQuorum Review - Independent Review Board
IRB Evaluation of Advertisements, Consent Forms and Study Tools

IRB Evaluation of Advertisements, Consent Forms and Study ToolsQuorum Review - Independent Review Board
Contenu connexe
Plus de Quorum Review - Independent Review Board
Webinar Slides: Biobanking & Future Research: Addressing the "Unknown" in the...

Webinar Slides: Biobanking & Future Research: Addressing the "Unknown" in the...Quorum Review - Independent Review Board
Webinar: Research Involving Subjects with Limited Capacity: IRB Expectations ...

Webinar: Research Involving Subjects with Limited Capacity: IRB Expectations ...Quorum Review - Independent Review Board
E consent for research: Considerations in Implementation and IRB Review

E consent for research: Considerations in Implementation and IRB ReviewQuorum Review - Independent Review Board
IRB Evaluation of Advertisements, Consent Forms and Study Tools

IRB Evaluation of Advertisements, Consent Forms and Study ToolsQuorum Review - Independent Review Board
Plus de Quorum Review - Independent Review Board (20)
Webinar Slides: Biobanking & Future Research: Addressing the "Unknown" in the...

Webinar Slides: Biobanking & Future Research: Addressing the "Unknown" in the...
Webinar: Research Involving Subjects with Limited Capacity: IRB Expectations ...

Webinar: Research Involving Subjects with Limited Capacity: IRB Expectations ...
E consent for research: Considerations in Implementation and IRB Review

E consent for research: Considerations in Implementation and IRB Review
IRB Evaluation of Advertisements, Consent Forms and Study Tools

IRB Evaluation of Advertisements, Consent Forms and Study Tools
Dernier
Dernier (20)
Top Quality Call Girl Service Kalyanpur 6378878445 Available Call Girls Any Time

Top Quality Call Girl Service Kalyanpur 6378878445 Available Call Girls Any Time
Call Girls Bareilly Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Bareilly Just Call 9907093804 Top Class Call Girl Service Available
Call Girls Gwalior Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Gwalior Just Call 9907093804 Top Class Call Girl Service Available
Call Girls Aurangabad Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Aurangabad Just Call 9907093804 Top Class Call Girl Service Available
Call Girls Nagpur Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Nagpur Just Call 9907093804 Top Class Call Girl Service Available
Premium Bangalore Call Girls Jigani Dail 6378878445 Escort Service For Hot Ma...

Premium Bangalore Call Girls Jigani Dail 6378878445 Escort Service For Hot Ma...
Call Girls Ludhiana Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Ludhiana Just Call 9907093804 Top Class Call Girl Service Available
Call Girls Bangalore Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Bangalore Just Call 9907093804 Top Class Call Girl Service Available
Top Rated Bangalore Call Girls Mg Road ⟟ 9332606886 ⟟ Call Me For Genuine S...

Top Rated Bangalore Call Girls Mg Road ⟟ 9332606886 ⟟ Call Me For Genuine S...
(Rocky) Jaipur Call Girl - 09521753030 Escorts Service 50% Off with Cash ON D...

(Rocky) Jaipur Call Girl - 09521753030 Escorts Service 50% Off with Cash ON D...
Pondicherry Call Girls Book Now 9630942363 Top Class Pondicherry Escort Servi...

Pondicherry Call Girls Book Now 9630942363 Top Class Pondicherry Escort Servi...
Call Girls Haridwar Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Haridwar Just Call 9907093804 Top Class Call Girl Service Available
Call Girls Coimbatore Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Coimbatore Just Call 9907093804 Top Class Call Girl Service Available
Top Rated Bangalore Call Girls Richmond Circle ⟟ 9332606886 ⟟ Call Me For Ge...

Top Rated Bangalore Call Girls Richmond Circle ⟟ 9332606886 ⟟ Call Me For Ge...
Call Girls Kochi Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Kochi Just Call 9907093804 Top Class Call Girl Service Available
The Most Attractive Hyderabad Call Girls Kothapet 𖠋 6297143586 𖠋 Will You Mis...

The Most Attractive Hyderabad Call Girls Kothapet 𖠋 6297143586 𖠋 Will You Mis...
Call Girls Varanasi Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Varanasi Just Call 9907093804 Top Class Call Girl Service Available
Best Rate (Patna ) Call Girls Patna ⟟ 8617370543 ⟟ High Class Call Girl In 5 ...

Best Rate (Patna ) Call Girls Patna ⟟ 8617370543 ⟟ High Class Call Girl In 5 ...
Lucknow Call girls - 8800925952 - 24x7 service with hotel room

Lucknow Call girls - 8800925952 - 24x7 service with hotel room
VIP Hyderabad Call Girls Bahadurpally 7877925207 ₹5000 To 25K With AC Room 💚😋

VIP Hyderabad Call Girls Bahadurpally 7877925207 ₹5000 To 25K With AC Room 💚😋
