Api List September
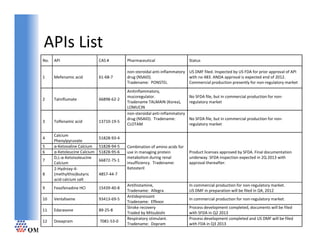
- 1. APIs List APIs List No. API CAS # Pharmaceutical Status non‐steroidal anti‐inflammatory US DMF filed. Inspected by US FDA for prior approval of API 1 Mefenamic acid 61‐68‐7 61 68 7 drug (NSAID). drug (NSAID) with no 483. ANDA approval is expected end of 2012. with no 483 ANDA approval is expected end of 2012 Tradename: PONSTEL Commercial production presently for non‐regulatory market Anitinflammatory, mucoregulator. No SFDA file, but in commercial production for non‐ 2 Talniflumate 66898‐62‐2 Tradename TALMAIN (Korea), regulatory market LOMUCIN non‐steroidal anti‐inflammatory drug (NSAID). Tradename: No SFDA file, but in commercial production for non‐ 3 Tolfenamic acid 13710‐19‐5 CLOTAM regulatory market Calcium 4 51828 93 4 51828‐93‐4 Phenylpyruvate 5 α‐Ketovaline Calcium 51828‐94‐5 Combination of amino acids for 6 α‐Ketoleucine Calcium 51828‐95‐6 use in managing protein Product licenses approved by SFDA. Final documentation D,L‐α‐Ketoisoleucine metabolism during renal underway. SFDA inspection expected in 2Q 2013 with 7 66872‐75‐1 Calcium insufficiency. Tradename: approval thereafter. y y 2‐Hydroxy‐4‐ Ketosteril 8 (methylthio)butyric 4857‐44‐7 acid calcium salt Antihistamine, In commercial production for non‐regulatory market. 9 Fexofenadine HCl 15439‐40‐8 Tradename: Allegra US DMF in preparation will be filed in Q4, 2012 Antidepressant 10 Venlafaxine 93413‐69‐5 In commercial production for non‐regulatory market. Tradename: Effexor Tradename: Effexor Stroke recovery Process development completed, documents will be filed 11 Edaravone 89‐25‐8 Traded by Mitsubishi with SFDA in Q2 2013 Respiratory stimulant. Process development completed and US DMF will be filed 12 Doxapram 7081‐53‐0 Tradename: Dopram with FDA in Q3 2013