Outermost quantum shell 3 - elements of Period 3
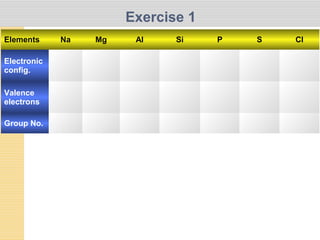
- 1. Exercise 1 Elements Na Mg Al Si P S Cl Electronic config. Valence electrons Group No.
- 2. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] config. 3s1 Valence electrons Group No.
- 3. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] config. 3s1 Valence 1 electrons Group No.
- 4. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] config. 3s1 Valence 1 electrons Group No. 1
- 5. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] [Ne] config. 3s1 3s2 Valence 1 electrons Group No. 1
- 6. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] [Ne] config. 3s1 3s2 Valence 1 2 electrons Group No. 1
- 7. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] [Ne] config. 3s1 3s2 Valence 1 2 electrons Group No. 1 2
- 8. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] [Ne] [Ne] config. 3s1 3s2 3s23p1 Valence 1 2 2+1=3 electrons Group No. 1 2 3
- 9. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] [Ne] [Ne] [Ne] config. 3s1 3s2 3s23p1 3s23p2 Valence 1 2 2+1=3 2+2=4 electrons Group No. 1 2 3 4
- 10. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] [Ne] [Ne] [Ne] [Ne] config. 3s1 3s2 3s23p1 3s23p2 3s23p3 Valence 1 2 2+1=3 2+2=4 2+3=5 electrons Group No. 1 2 3 4 5
- 11. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] [Ne] [Ne] [Ne] [Ne] [Ne] config. 3s1 3s2 3s23p1 3s23p2 3s23p3 3s23p4 Valence 1 2 2+1=3 2+2=4 2+3=5 2+4=6 electrons Group No. 1 2 3 4 5 6
- 12. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] [Ne] [Ne] [Ne] [Ne] [Ne] [Ne] config. 3s1 3s2 3s23p1 3s23p2 3s23p3 3s23p4 3s23p5 Valence 1 2 2+1=3 2+2=4 2+3=5 2+4=6 2+5=7 electrons Group No. 1 2 3 4 5 6 7
- 13. Exercise 1 Elements Na Mg Al Si P S Cl Electronic [Ne] [Ne] [Ne] [Ne] [Ne] [Ne] [Ne] config. 3s1 3s2 3s23p1 3s23p2 3s23p3 3s23p4 3s23p5 Valence 1 2 2+1=3 2+2=4 2+3=5 2+4=6 2+5=7 electrons Group No. 1 2 3 4 5 6 7 Outermost quantum shell = 3 the principal quantum number of the valence shell is 3 all the elements in this period belong to Period 3