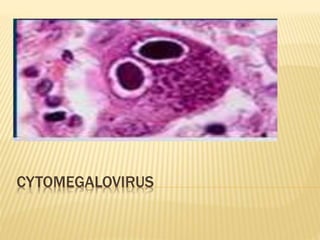
Cytomegalovirus
- 2. HISTORY First isolated in 1956 from 2 dying infants from salivary gland and kidneys with demonstration of inclusion bodies “Salivary Gland Virus” 1960 Wellar et al proposed name ‘cytomegalovirus’ CMV first isolated from renal transplant patient in 1965
- 3. CMV (CYTOMEGALOVIRUS) Human herpes virus type 5 (HHV-5) The largest in Herpesvirus Immunobiology of human cytomegalovirus Clin Microbiol Rev. 2009 Jan;22(1):76-98 http://www.iayork.com CMV Structure Double-stranded DNA core Icosahedral nucleocapsid Tegument (proteinaceous matrix) Lipid bilayer envelope contains Glycoproteins
- 5. EPIDEMIOLOGY Seroprevalence of CMV in the human population: 30~90% in developed countries and may be higher in developing countries, increasing with age. Transmitted via: Saliva Sexual contact Placental transfer Breastfeeding Blood transfusion Solid-organ transplantation Hematopoietic stem cell transplantation
- 6. IMMUNE CONTROL Generally, CMV infection is held in check by the host’s immune response. Primary CMV infection in an immunocompetent host is normally asymptomatic. → CMV disease is generally restricted to the immunocompromised host. CMV has the ability of lifelong persistent, latent infection, and can reactivate under certain conditions. → In transplant recipients, infection with CMV from the donor organ or the reactivation of CMV in the recipient can lead to disease development.
- 7. IMPORTANCE OF CMV INFECTION In the absence of any form of antiviral prophylaxis, CMV disease occurs in 20–30% of transplant recipients, with a vast majority of the episodes developing within 90 days post-transplant. The single most important infectious complication after solid organ transplant(SOT). A major complication of hematopoietic stem cell transplantation(HSCT). ⇒ CMV is a major cause of morbidity in transplant recipients.
- 8. CMV - PATHOGENESIS WBCs and CD 13 + are reservoir cells Detected in most tissues of body and remains latent Enter host cell by fusion or phagocytosis Viral particles are made and assembled in nucleus , attain envelope by budding through inner nuclear membrane Replication produces immediate early (IE), early and late antigens IE (nucleus) : direct production of viral and cellular genes Early (cytoplasm) : directs viral DNA synthesis Late ( Nucleus + Cytoplasm) : directs production of structural nucleocapsid proteins Antivirals interupt DNA synthesis
- 9. ALSO ….. IE gene upregulates transcription and expression of IL - 2 and IL- 2 receptor IE gene prevents inhibitory effect of cyclosporine on IL – 2 gene transcription IE and early antigens also upregulate adhesion molecules such as ICAM –1 and LFA – 3, further increased by antiviral agents CMV has ability to down regulate MHC class 1 molecules
- 10. CMV AND SOLID ORGAN TRANSPLANTATION • CMV is still among the most important infectious complications after transplant • In the absence of prophylaxis, CMV reactivation can occur in over 75% of solid organ transplant recipients depending on other risk factors(1) • Once CMV infection is established, then its replication is highly dynamic with rapid increases in viral load CMV infection may lead to tissue invasive disease 10 1. Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med.
- 11. CMV INFECTION: RISK CATEGORIES IN SOLID ORGAN TRANSPLANT RECIPIENTS Risk Category Donor (D) or Recipient (R) Seropositivity (+/-) High D+/R- Intermediate* D+/R+, D-/R+ Low D-/R- 11 * D+/R+ generally at higher risk than D-/R+ Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation – challenging the status quo. Clinical Transplantation. 2007;21:149-158.
- 12. OTHER RISK FACTORS Type of organ – Lung/small intestines > pancreas, heart > liver, kidney – Due to transplanted load; immune response in the allograft; level of immunosuppression Intensity of immunosuppression – Antilymphocyte products (e.g., thymoglobulin) – Dose, duration, and overall intensity of drugs – Newer agents – alemtuzumab, others? 12
- 13. CMV PATHOGENESIS • Viral factors – replication dynamics – immune evasion – viral heterogeneity – viral co-infections • Host factors – CD4+, CD8+ T-cell – NK cell, B-cell – exogenous immunosuppression – D/R immune status 13
- 14. INFLAMMATION (CYTOKINES, NF-B) LATENT CMV INFECTION ANTILYMPHOCYTE ANTIBODIES OTHER HERPES VIRUSES SEPSIS/ SURGERYREJECTION 14
- 15. CMV INFECTION Latent CMV infection Active CMV infection (viral replication) Direct effects Indirect effects 15
- 16. DIRECT EFFECTS OF CMV INFECTION CMV Viral Syndrome • Fever, malaise, myalgias • Leukopenia, thrombocytopenia, and other laboratory abnormalities Tissue Invasive Disease • Hepatitis • Pneumonitis • Colitis • Carditis • Nephritis • Pancreatitis • Retinitis Direct Effects 16
- 17. INDIRECT EFFECTS OF CMV INFECTION Altered host immune response • Graft rejection; graft dysfunction • Opportunistic infections: Bacterial fungal superinfection • Decreased graft and patient survival • Herpesvirus interactions: EBV/PTLD Indirect Effects 17
- 18. Infection in Solid-Organ Transplant Recipients N Engl J Med 2007;357:2601-14 CLINICAL FEATURES Cellular effects: MHC, cytokine expression “indirect effects” CMV disease “direct effects” CMV Syndrome (fever, myalgia,,) End-organ Disease (pn, colitis, retinitis,,,) Allograft injury Allograft rejection EBV- associated PTLD Opportu- nistic infections Atherosclerosis, BO, etc Active CMV infection (viremia and tissue infection) Latent CMV infection
- 19. Management of CMV infection and Disease in Transplant Patients Herpes. 2004 Dec;11(3):77-86 www.mdconsult.com
- 20. CMV HEPATITIS 20
- 21. Chorioretinitis after liver transplantation Transpl Infect Dis 2008:10:27-43 The right eye showed diffuse fundus blurring with visual loss, The left eye presented with cotton-wool spots and hemorrhage. CLINICAL FEATURES - RETINITIS
- 22. Cytomegalovirus Pneumonia After Stem Cell Transplantation AJR 2006; 187:W636–W643 Small ill-defined centrilobular opacities Patchy GGO and septal edema CLINICAL FEATURES - PNEUMONIA
- 23. CLINICAL FEATURES - COLITIS
- 24. CMV AND KIDNEY Glomerular Disease Tubulointerstial Disease Kidney transplant rejection with or without glomerular involvement
- 25. CMV AND KIDNEY Nephrotic syndrome due to MN especially in infants and children with primary CMV syndrome Necrotising and proliferative glomerulonephritis Adult Ig A Nephropathy – association not fully understood HSP Type II Cryoglobulinemia Type I MPGN Collapsing FSGS Thrombotic Microangiopathy Transplant Glomerulopathy Tubular inclusions + interstial infiltrate have been seen with bone marrow transplant patients
- 26. CMV - DIAGNOSIS A. seroconversion with the appearance of anti-CMV IgM antibodies B. a fourfold increase in preexisting anti-CMV IgG titers C. detection of CMV antigens in infected cells D. detection of CMV-DNAemia by molecular techniques E. isolation of the virus by culture of the throat, buffy coat, or urine.
- 27. CMV DIAGNOSIS Diagnosis CMV disease: acute symptomatic + evidence of CMV infection 1.Quantitative CMV DNA by PCR diagnosis and monitor response 2.Qualitative CMV DNA detection by PCR extremely sensitive, can’t diff active or latent 3.CMV pp65 antigen: in peripheral lymphocyte semiquantitative fluorescent assay more rapid > C/S
- 28. CMV DIAGNOSIS 4.Tissue culture: delay diagnosis 5.Serum CMV IgM IgG Ab: good for screening before KT but not sensitive for diagnosis 6 Histopathology: delay diagnosis and inadequate specimen collection
- 29. Characterized by intra-nuclear inclusion with enlargement of both cell & nucleus. Cytoplasmic inclusions are also seen in the infected cells.
- 30. There are foci of concentrated inflammatory infiltrate in renal parenchyma
- 31. Enlarged CMV-infected renal tubular epithelial cells with prominent intranuclear inclusion surrounded by a clear halo (bull-eye appearance).
- 32. CMV-infected renal tubular epithelial cell with typical bull-eye appearance. Note the rather enlarged CMV-infected cell as compared to adjacent normal renal tubular epithelial cells.
- 33. Time of Presentation of Common Viral Illnesses Post-Transplant Viral Infection after Renal Transplantation Clin J Am Soc Nephrol 3: S76–S86, 2008
- 34. Improving Outcomes for Solid-Organ Transplant Recipients At Risk from Cytomegalovirus Infection: Late-Onset Disease and Indirect Consequences Clinical Infectious Diseases 2008; 46:732–40 Five-year follow-up data for patients with persistent cytomegalovirus (CMV) infection of the graft, showing graft survival in patients with persistent CMV infection, compared with patients with nonpersistent or no CMV infection in the graft. CLINICAL FEATURES – GRAFT SURVIVAL CMV(-) CMV(+)
- 35. CMV TREATMENT In immunocompetent hosts the disease may be asymptomatic and no treatment may be required Tapering of immunosuppresants may be required mononucleosis-like syndrome may resolve without the administration of antiviral drugs Invasive disease need anti-viral drugs
- 36. IMMUNOSUPPRESIVE THERAPY azathioprine or mycophenolate mofetil should be given in reduced dosage or discontinued. Reduced immunotherapy is necessary when there is evidence of tissue invasive disease and organ involvement (including chorioretinitis). cyclosporine or tacrolimus should be discontinued in this setting remains controversial. We usually do not discontinue cyclosporine or tacrolimus unless there is evidence of life- threatening infection Corticosteroids are generally continued to prevent possible adrenal insufficiency.
- 37. ANTI-CMV THERAPY Ganciclovir: (Cytovene® and Cymevene®) is a synthetic analogue of 2-deoxyguanosine, that competitively inhibits the incorporation of dGTP by viral DNA polymerase–intravenous or oral Valganciclovir: (Valcyte®) a prodrug form of ganciclovir with improved oral bioavailability Foscarnet: (Foscavir) is an inhibitor CMV DNA polymerase (UL54) ‒ Useful for ganciclovir resistant CMV ‒ Major limitation is nephrotoxicity Cidofovir: (Vistide) inhibits viral DNA polymerase ‒ May be useful for ganciclovir resistant CMV but not well studied in organ transplant recipients Maribavir: is an investigational agent that prevents viral encapsidation and nuclear egress 37
- 38. CMV TREATMENT • Mild to moderate CMV disease: oral valganciclovir = i.v. ganciclovir • Severe CMV disease: - high CMV viral load > 5 x 105 copies/ml - severe tissue invasive disease - fail achieve viral load reduction after D7 of oral valganciclovir
- 39. ganciclovir 5 mg/kg every 12 hours until viremia suppression (usually 14-21 days) Blood for viral load at least q 1 week Prior to initial treatment need to be excluded. As an example, CMV infection may be associated with Pneumocystis carinii pneumonia, • if ongoing risk for CMV : long term maintenance - oral ganciclovir 1 g tid - valganciclovir 450-900 mg OD
- 40. CMV PREVENTION • Pre-emptive – Guided by laboratory monitoring for evidence of early viral replication; treatment is started when CMV viral load or antigenemia reaches a certain threshold • Universal prophylaxis – Therapy from the time of transplant to all patients or a subgroup of patients at risk for CMV disease 40
- 41. + + + + + __ + + _ 0 4 8 12 weeks Initiate pre-emptive therapy to prevent CMV disease CMV disease TEST PRE-EMPTIVE THERAPY __ 41
- 42. PRE-EMPTIVE THERAPY • Advantages: – Minimizes drug exposure – This may potentially decrease toxicity and costs – Theoretically lower risk of resistance – Less late-onset disease: may allow development of cell-mediated immune response • Disadvantages: – Logistically more difficult to coordinate – May be unsuccessful in preventing progression to active disease in high-risk patients due to rapid doubling time – May not eliminate the indirect effects of CMV 42
- 43. MODELING THE DYNAMICS OF CMV REPLICATION Log10ViralLoad Time (days) Doubling time = ln2/a y = y0eax Average DT= 1-2 days Detection threshold 43Emery V, et al. Lancet. 2000;355(9220):2032-2036.
- 44. ANTIVIRAL PROPHYLAXIS • Antiviral therapy from the time of transplant to all patients or a subgroup of patients (universal or targeted) • Advantages: – Proven efficacy – Decreases indirect effects – Ease of administration • Disadvantages: – Drug toxicity, cost – Resistance – Late-onset CMV disease 44
- 45. 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 CMV Disease CMV Syndrome CMV Invasive Organ Disease RelativeRisk CMV PROPHYLAXIS Hodson, et al. Cochrane Database Sys Rev. 2008;2:CD003774. 0.42 0.34 0.41 45
- 46. EFFECTS OF ANTI-CMV PROPHYLAXIS ON CONCOMITANT INFECTIONS 46 Hodson EM, et al. Lancet. 2005;365:2105-2115.
- 47. LATE ONSET CMV DISEASE DEFINITION • CMV disease occurring > 3 months post transplant • May be primary infection (D+/R-) or recurrence (R+) • In epidemiology studies associated with significant morbidity (including graft dysfunction) and occasional mortality (indirect effects)(1) • Incidence 3%-17%; In IMPACT study 37% with 3 months of prophylaxis in D+/R- 47 Limaye, AP, et al. Transplantation. 2004;78(9):1390-1396.
- 48. CMV PROPHYLAXIS: LATE-ONSET DISEASE Prophylaxis period PatientsWithNoCMVDisease(%) 0 10 20 30 40 50 60 70 80 90 100 Time (days) 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 364 D+/R- SOT patients Ganciclovir (oral) Valganciclovir Paya, et al. Am J Transplant. 2004;4:611-620.48
- 49. PRE-EMPTIVE VS. PROPHYLAXIS There are very few comparative randomized trials comparing pre-emptive therapy vs. prophylaxis Khoury et al: 98 kidney transplant patients randomized to pre-emptive therapy (valganciclovir) vs. prophylaxis (valganciclovir 100 days) ‒ Equally effective in preventing CMV disease Kliem et al: randomized 148 renal transplant patients to pre-emptive therapy (I.V. ganciclovir) vs. prophylaxis (3 months oral ganciclovir) ‒ Long-term graft survival at 4-years post transplant was significantly improved in the prophylaxis group 49 Khoury JA, et al. Am J Transplant. 2006;6(9):2134-2143. Kliem V, et al. Am J Transplant. 2008;8(5):975-983.
- 50. Prophylaxis Pre-emptive Evidence of efficacy +++ ++ Indirect effects/mortality ++ + Other viruses + for some ? Ease ++ +/- Late onset disease ++ - Resistance Low Very Low PROPHYLAXIS VS. PRE-EMPTIVE THERAPY 50
- 51. WHAT ABOUT PROLONGING PROPHYLAXIS? • Potential benefits – decrease disease – improve graft outcomes • Potential pitfalls – push disease further? – cost – toxicity 51
- 52. THE IMPACT TRIAL • Kidney recipients, D+/R-, N=316 • 3 months of valganciclovir + 3 months placebo vs. 6 months of valganciclovir • 900 mg QD dose adjusted • Incidence of CMV disease at 12 and 24 months post transplant 52 Humar A, et al. Am J Transplant. 2009;9(suppl2):248. Abstract 201.
- 53. DESIGN Valganciclovir 900 mg od* Valganciclovir 900 mg od* Valganciclovir 900 mg od* Placebo 100 days 200 daysRandomization 12 months post transplant VGCV-100 days: VGCV-200 days: * dose adjusted for renal function Humar A, et al. Am J Transplant. 2009;9(suppl 2):248. Abstract 201.53
- 54. CONFIRMED CMV DISEASE 36.8 16.1 0 10 20 30 40 50 VGCV-100 days VGCV-200 days Proportionofpatientswithconfirmed CMVdiseaseat12months(%) p < 0.0001 * Patients without an assessable month 12 status are not assumed to have the event in this sensitivity analysis. Humar A, et al. Am J Transplant. 2009;9(suppl 2):248. Abstract 201.54
- 55. INCIDENCE OF CONFIRMED CMV DISEASE 0 0.4 0.2 0 0.8 0.6 18060 120 240 360 1.0 300 Event-freeprobability Study day Number of patients left VGCV-100 days 163 161 161 157 151 125 110 104 102 101 95 94 83 4 VGCV-200 days 155 154 152 150 149 147 145 143 136 130 125 122 120 7 VGCV 200 days VGCV 100 days Humar A, et al. Am J Transplant. 2009;9(suppl 2):248. Abstract 201.55
- 56. CMV TREATMENT • Treat with full dose IV ganciclovir or oral valganciclovir (VICTOR trial)(1) • Monitor CMV viral load or antigenemia once weekly while on therapy • Generally, continue treatment until undetectable • May consider a 1-3 month course of secondary prophylaxis after completion of treatment 561. Ashberg A, et al. Am J Transplant. 2007;7(9):2106-2113.
- 57. ORAL VS. IV THERAPY OF CMV VICTOR TRIAL Maintenance Day 21 to 49 Follow-up Phase Month 3 to 12 Induction Day 0 to 20 Oral valganciclovir 900 mg BID Oral valganciclovir 900 mg QD No study medicationCMV Disease IV ganciclovir 5 mg/kg BID • Multicenter non-inferiority study • 42 centers: 25 in Europe, 9 in Latin America, 4 in India, 2 in Canada, 2 in Australia and New Zealand Asberg, et al. AJT. 2007;7(9):2106-2113.57
- 58. CMV DRUG RESISTANCE DIAGNOSIS • Increases of viral load as surrogate marker for resistance – drug naïve subjects, early during treatment, low-risk setting (R+) • drug resistance unlikely • increases most likely due to the underlying immunosuppression – after significant exposure (especially low dose), high-risk setting • more likely • true viral load increase >0.5 log10 (>3x baseline) • Testing: direct genotypic testing if resistance is suspected – UL97 gene: ganciclovir – UL 54 gene: foscarnet, cidofovir, ganciclovir (high level) 58
- 59. CMV RESISTANCE: PROPOSED MANAGEMENT ALGORITHM 59 At least 2 weeks of adequate dose of ganciclovir with increasing or unchanged viral load Reduce immunosuppression. Send for genotypic resistance testing Severe CMV disease Non-severe CMV disease Switch to or add foscarnet at full dose Increase ganciclovir dose up to 10 mg/kg BID or ½ dose ganciclovir / ½ dose foscarnet Alter therapy based on genotypic resistance testing and clinical response. Adjunctive unproven therapy may be required.
- 60. NEW DRUGS? MARIBAVIR Maribavir – Inhibits CMV UL97: prevents viral encapsidation and nuclear egress – Potent in vitro activity against CMV – Phase 3 halted OLTx, BMT due to failure primary endpoint 60
- 61. WHAT DOES THE FUTURE HOLD? • Use of translational research to establish better predictive tools – host response: CD8, CD4 responses to specific herpesvirus antigens – viral factors: viral gene expression – understand the impact of herpesvirus interactions • Novel targets: tailored drug therapy, selective immunosuppression, immunosuppression with co-existing antiviral activity • Novel preventive strategies – vaccine strategies: DNA vaccine, multi-epitope vaccines – cell-mediated therapeutic modalities 61
