Case record...Cerebral amyloid angiopathy (CAA),
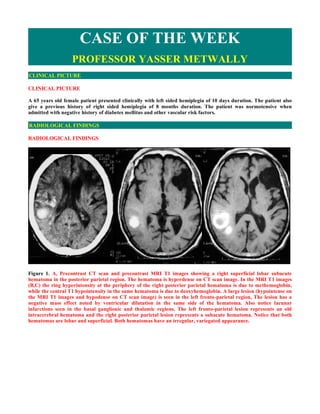
- 1. CASE OF THE WEEK PROFESSOR YASSER METWALLY CLINICAL PICTURE CLINICAL PICTURE A 65 years old female patient presented clinically with left sided hemiplegia of 10 days duration. The patient also give a previous history of right sided hemiplegia of 8 months duration. The patient was normotensive when admitted with negative history of diabetes mellitus and other vascular risk factors. RADIOLOGICAL FINDINGS RADIOLOGICAL FINDINGS Figure 1. A, Precontrast CT scan and precontrast MRI T1 images showing a right superficial lobar subacute hematoma in the posterior parietal region. The hematoma is hyperdense on CT scan image. In the MRI T1 images (B,C) the ring hyperintensity at the periphery of the right posterior parietal hematoma is due to methemoglobin, while the central T1 hypointensity in the same hematoma is due to deoxyhemoglobin. A large lesion (hypointense on the MRI T1 images and hypodense on CT scan image) is seen in the left fronto-parietal region, The lesion has a negative mass effect noted by ventricular dilatation in the same side of the hematoma. Also notice lacunar infarctions seen in the basal ganglionic and thalamic regions. The left fronto-parietal lesion represents an old intracerebral hematoma and the right posterior parietal lesion represents a subacute hematoma. Notice that both hematomas are lobar and superficial. Both hematomas have an irregular, variegated appearance.
- 2. Figure 2. Precontrast MRI T1 images (A,B) and MRI T2 image (C). The precontrast T1 hyperintensity and T2 hyperintensity is due to Extracellular migration of methemoglobin. The hypointense rim surrounding the right posterior parietal lesion and the left fronto-parietal lesion on the MRI T1,T2 images is due to hemosiderin. Both hematomas have an irregular, variegated appearance. Figure 3. Bilateral hematomas of different ages. MRI T2 images showing a subacute right posterior parietal hematoma and an old left fronto-parietal hematoma. The T2 hyperintensity is due to extracellular migration of methemoglobin. The hypointense rim surrounding the right posterior parietal lesion and the left fronto-parietal lesion on the MRI T2 images is due to hemosiderin. The older left fronto-parietal hematoma is still surrounded by the hemosiderin hypointense rim. Both hematomas have an irregular, variegated appearance.
- 3. Figure 4. Bilateral hematomas of different ages. MRI T2 images showing a subacute right posterior parietal hematoma and an old left fronto-parietal hematoma. The T2 hyperintensity is due to extracellular migration of methemoglobin. The hypointense rim surrounding the right posterior parietal lesion and the left fronto-parietal lesion on the MRI T2 images is due to hemosiderin. The older left fronto-parietal hematoma is still surrounded by the hemosiderin hypointense rim. Both hematomas have an irregular, variegated appearance. Figure 5. CT scan (A) and precontrast MRI T1 (B,C) and MRI T2 image (D) showing the right posterior parietal subacute hematoma and the left old fronto-parietal hematoma
- 4. The hematoma biochemical stages Table 1. The MRI biochemical stages of cerebral hematomas [25] Biochemical substance MRI changes Oxyhemoglobin Oxyhemoglobin lacks unpaired electrons and thus clot signal is close to normal brain parenchyma- normal to slightly lower signal on TI-weighted images and slightly higher signal on T2-weighted images Paramagnetic intracellular Because the deoxyhemoglobin within intact, clotted hypoxic red blood cells deoxyhemoglobin. does not cause T1 shortening, the hematoma will have normal to slightly lower signal on TI-weighted MR images. The concentration of red blood cells with clot and the concentration of fibrin cause T2 shortening, with areas of very low signal on T2-weighted spin echo and T2 * -weighted gradient echo images Paramagnetic intracellular Proton-electron dipole-dipole interactions between hydrogen atoms and the methemoglobin. paramagnetic centers of methemoglobin will cause marked TI shortening and very high signal intensity on TI-weighted images within the periphery of the hematoma. The intracellular methemoglobin will cause T2 shortening and very low signal on T2-weighted images. Extracellular migration of MR will exhibit the persistent high signal of extracellular methemoglobin on methemoglobin. TI - and T2-weighted images for up to a year. The peripheral rim of hemosiderin and ferritin has slightly low signal on Tl- and marked low signal on T2-weighted images from the susceptibility effect of hemosiderin within macrophage lysosomes. Clot resorption begins from the Focal atrophy is characterized by a decrease in the size of cortical gyri, with periphery inward, and depending compensatory enlargement of cerebrospinal fluid spaces and dilatation of on the size of the hematoma, may the adjacent ventricle. Cystic cavities are surrounded by gliosis and vary from one to six weeks in hemosiderin scarring. duration. Necrotic tissue is sloughed and cystic cavities are formed over the next 6 to 12 months. The multiple intracerebral hematomas are due to amyloid angiopathy because of
- 5. 1- The multiplicity of the lesions. 2- The superficial nature of both hematomas. 3- The lobar location of both hematomas. 4- Both hematomas have an irregular, variegated appearance. 5- Absence of vascular risk factors. DIAGNOSIS: DIAGNOSIS: AMYLOID ANGIOPATHY WITH MULTIPLE LOBAR INTRACEREBRAL HEMATOMAS DISCUSSION DISCUSSION Cerebral amyloid angiopathy Cerebral amyloid angiopathy (CAA), also known as congophilic angiopathy, affects exclusively the cerebral vasculature, without involvement of other areas of the body. The amyloid substance is deposited in the media and adventitia of small and medium diameter arteries, as well as in veins, of the cortical surface and leptomeninges. The histological diagnosis is made by showing areas of the vessel wall that stain with Congo red and show a characteristic apple green birefringence under polarized light. CAA characteristically affects the elderly, with a linear increase in frequency with age. In routine autopsy studies, the frequency of CAA has been 5-13% in those patients aged 60-69, 20-40% in patients aged 70-79, 35-45% in patients aged 80-89, and 45-58% in individuals older than 90 years of age. The main clinical manifestation of CAA is ICH, but an association with Alzheimer's disease and with a leukoencephalopathy are now well recognized as well. [25] Figure 6. Lobar hemorrhage due to amyloid angiopathy, B Microscopic section of the brain cortex, section has been stained with Congo Red for amyloid viewed with polarized light. The section shows relatively preserved cortical neurons and the blood vessels shows birefringence with polarized light. In some areas the walls of the blood vessels are yellow-green (arrow). Diagnosis: Amyloid angiopathy, also known as congophilic angiopathy.
- 6. The ICHs that occur in the setting of CAA have several characteristic features. These include: [25] A lobar location, due to the cortical and leptomeningeal distribution of the angiopathy. CCA A frequently irregular, variegated appearance on a computed tomography (CT) scan, which results from extension of the superficially-located haemorrhage into the adjacent subarachnoid space. CCA A tendency to be recurrent, on occasion with multiple episodes of lobar ICH over periods of months or CCA years, a feature that is exceptionally rare in ICH due to hypertension. CCA The occasional presence of multiple simultaneous haemorrhages, is also a distinct rarity in ICH of hypertensive mechanism. The ICH in patients with CAA can occasionally be related to preceding head trauma or a neurosurgical procedure, raising the possibility that mechanically-induced vascular rupture may be at times involved in its pathogenesis. Similarly, the use of anticoagulant and fibrinolytic agents is being increasingly suspected as a potential risk factor in instances of ICH in the elderly, presumed to harbour CAA as a local vascular lesion predisposing to ICH. The actual mechanism of bleeding in CAA has not been elucidated. A factor that may be related to bleeding is the association of this angiopathy with other vascular changes, notably fibrinoid necrosis. Such vascular lesion has been found with high frequency (71%) in instances of CAA associated with ICH, while it is not present in patients with CAA but without ICH. These data suggest that despite the high prevalence of CAA in the elderly, the relatively low frequency of ICH may be explained by the need to have the associated changes of fibrinoid necrosis in the affected vessels, in order to result in rupture and ICH. [25] CA with ICH is a sporadic condition, but two rare familial forms have been described only in specific geographical locations in Iceland and in Holland. The latter form of familial CAA with ICH has been characterized as a biochemical abnormality in the precursor protein of amyloid, which is also present in Alzheimer's disease and Down's syndrome. Other associations of CAA include Alzheimer's disease and a leukoencephalopathy. The histological features of Alzheimer's disease are present in approximately 40% of patients with CAA-related ICH, and 30-40% of patients with CAA have clinical features of dementia. The leukoencephalopathy of CAA affects the white matter of the cerebral hemispheres, with preservation of the 'U' fibres, corpus callosum, internal capsule, optic radiation, and white matter of the temporal lobes. The imaging diagnosis of this leukoencephalopathy is greatly facilitated by the use of magnetic resonance imaging (MRI), which shows hyperintensity of the white matter in T2-weighted sequences. [25] NEUROIMAGING OF HEMATOMA Neuroimaging of cerebral hematoma CT imaging of haematoma. A cerebral haematoma, in the acute stage, has higher attenuation values on precontrast scan (hyperdense). The higher attenuation values of fresh blood is due to the existence of packed haemoglobin in the haematoma. In particular the globin component of the haemoglobin is responsible for the increased CT density on precontrast scan. With progressive absorption of haemoglobin, (this usually starts from the periphery of the haematoma) the attenuation value of the haematoma gradually decreases until the high density haematoma is replaced by a low density space occupying cyst.
- 7. Figure 7. A, Acute haematoma, B, an apoplectic cyst and C, an old haematoma (slit-like scar) The evolution of the haematoma from a high density clot to a low density cyst usually takes a period that ranges between one month to three months. The walls of this cyst might enhance and the haematoma at this stage might be mixed with abscess or glioma. History is of paramount significance at this stage. Very old haematoma appears by CT scan as a slit-like hypodense area with negative mass effect. In general Haematomas are space-occupying with positive mass effect and are commonly surrounded by a hypodense oedema area. The most common sites are the putameno-capsular and the thalamic sites and either of them might rupture intraventricularly. Less common sites includes the parietal lobe, pons and cerebellum. [25] The diagnosis of acute ICH is virtually 100% reliable with non-contrast CT due to the characteristic mass of blood of high attenuation value, due to the presence of the globin component of the haemoglobin molecule. Under exceptional circumstances, patients with profound anaemia, with a haematocrit of 20% or less have presented with an acute haematoma which was isointense to brain on account of the low haemoglobin contents of the fresh haematoma. Fresh blood has an attenuation value of 55-85 Hounsfield units, the high attenuation (50-70 Hounsfield units) is from high protein concentration within intact red blood cells and not iron content. [25] As the fresh clot starts to retract after 24-48 hours from onset, there is serum extrusion around its periphery, resulting in a ring of hypointensity that surrounds the haematoma . In the subacute stage, the haematoma maintains its mass effect but becomes progressively less dense, from the periphery toward the center, until reaching isointensity with the adjacent brain parenchyma. The infusion of intravenous contrast at this stage can demonstrate an area of ring enhancement at the periphery of the haematoma. In the chronic stage, the mass effect of the haematoma is no longer present, post-contrast enhancement has disappeared after about 6 weeks from onset , and the residual is a hypointense cavity, at times in the form of a slit that can be indistinguishable from an area of old cavitated infarction. [25] More detailed description of the CT scan appearance of brain hemorrhage The CT appearance of hemorrhage is determined by the degree of attenuation of the x-ray beam, which is proportional to the density of hemoglobin protein (relative to plasma concentration) within the hematoma. [25] Immediately following vessel rupture, the hematoma consists of a collection of red blood cells, white blood cells, platelet clumps, and protein-rich serum that has a heterogeneous appearance on CT with attenuation in the range of 30–60 Hounsfield units (HU), depending on the degree of plasma extrusion. In this hyperacute phase, hemorrhage may be difficult to distinguish from normal cortex because of similar attenuation. Over minutes to hours, a fibrin clot forms with an increase in attenuation to 60–80 HU (Fig. 8). Clot retraction and extrusion of serum can further increase attenuation to as high as 80–100 HU in the center of the hematoma. The degree of attenuation may be reduced in patients with severe anemia, impaired clot formation due to coagulopathy, or volume averaging with adjacent tissue. Vasogenic edema evolves around the hematoma within hours and may continue to increase for up to 2 weeks after hemorrhage onset. [25]
- 8. Figure 8. CT appearance of hemorrhage. Serial CT scans of right thalamic hematoma. (A) Acute ICH in the right thalamus with mean attenuation 65 HU. (B) CT performed 8 days later than (A); the periphery of the hematoma is now isodense to the brain while the center of the hematoma has mean attenuation 45 HU. (C) CT performed 13 days later than (A) shows continued evolution of the hematoma with decreasing attenuation. (D) CT performed 5 months later than (A) shows a small area of encephalomalacia in the location of the previous hemorrhage. Over the following days, cells and protein are broken down and scavenged by macrophages, leading to slowly decreasing attenuation, with the greatest decrease at the periphery of the hematoma and more gradual evolution toward the center (Fig. 9). Within 4 to 9 days, the hematoma attenuation decreases to that of normal cortex, and within 2 to 3 weeks to that of normal white matter. The CT recognition of subacute intracerebral hematoma can be challenging because the attenuation is similar to that of normal brain tissue, although mass effect may still be present. MR imaging can confirm subacute hematoma. As time goes on, attenuation continues to decrease to levels below that of the normal brain. Eventually, the hematoma resolves into a fluid-filled or slit-like cavity that may be difficult to visualize on CT (Fig. 9). Contrast enhancement is not present in the initial days following ICH but may develop at the periphery in weeks to months, sometimes leading to diagnostic confusion with brain tumor or abscess. A blood-fluid level may be seen in medium to large ICH within the first hours after onset; the dependent portion displays higher attenuation due to sedimentation of cellular elements. This finding may be more common in ICH caused by anticoagulation, but it is not specific and has also been described in ICH due to hypertension, trauma, tumor, or arterial-venous malformation. The association with shorter time interval from ICH onset, and in some cases with anticoagulation, has led to speculation that incomplete clotting is required for blood-fluid level formation. Figure 9. CT with blood-fluid level. A 77-year-old woman was admitted with coma of 4 hours' duration. CT scan shows massive left hemispheric hematoma with blood-fluid level. No history of anticoagulation or coagulopathy. Box 1. As the hemorrhage evolves, different characteristic appearances can be identified on CT, depending on the age of the bleed. CT findings over time are as follows:
- 9. After 7-10 days, the high density of blood begins to decrease, starting from the periphery of the lesion. From 1-6 weeks, peripheral enhancement can be seen, mimicking the appearance of an abscess, possibly related to hypervascularity at the periphery of a resolving hematoma or disruption of the blood-brain barrier. By 2-4 months, decreased density indicates cavity formation. A residual cavity is the final stage, which is reached after complete absorption of necrotic and hemorrhagic tissue. MR imaging of cerebral hematoma Physical Factors The appearance of intracranial hemorrhage on MR imaging is influenced by intrinsic factors, such as age, location, and cause of the hemorrhage, as well as extrinsic factors, which include the strength of the magnetic field and the pulse sequence parameters used for imaging. For the purposes of this discussion, the appearance of hemorrhage is examined in five time frames: hyperacute (O to 12 hours), acute (12 hours to 3 days), early subacute (3 days to 1 week), late subacute (1 week to months), and chronic (months to years). Magnetic susceptibility refers to the ability of tissues within a magnetic field to generate their own local magnetic field. When a substance generates a local magnetic field that augments the main magnetic field, it is termed paramagnetic. The number of unpaired electrons within the outer shell of atoms or molecules is responsible for the paramagnetic effect. The greater the number of unpaired electrons, the larger the effect. In the normal afferent cerebral circulation, oxyhemoglobin, which is non paramagnetic (diamagnetic), predominates. When oxyhemoglobin is isolated from an oxygen-rich environment, progressive changes occur that yield three paramagnetic products of hemoglobin: deoxyhemoglobin, followed by methemoglobin, and then hemosiderin. The paramagnetic effects of these three hemoglobin byproducts are in large part responsible for the distinctive MR imaging appearance of the various stages of intracranial hemorrhage, particularly at high field strength (1.5 T). Paramagnetic substances alter the local magnetic environment experienced by individual hydrogen nuclei. When placed within an external magnetic field (the MR scanner), paramagnetic substances generate their own local magnetic field, which is additive to the externally applied field. This situation induces magnetic field inhomogeneity, which affects MR relaxation processes, causing accelerated signal loss because of spin dephasing and loss of transverse coherence (enhanced T2 relaxation). This accelerated signal loss results in decreased signal intensity on T2-weighted images roughly proportional to the square of the field strength of the MR scanner. Therefore, high field MR scanners (1.5 T) are more sensitive for the detection of hemorrhage than low field scanners MR appearance of intracerebral hemorrhage The physics of MR imaging of hemorrhage is complex; multiple reviews have covered this topic in detail [1,2]. A brief explanation is warranted here, however, because an understanding of the signal characteristics of hemorrhage, as well as their evolution over time, is essential for radiologic interpretation. The MR signal intensity of hemorrhage is dependent on both the chemical state of the iron atoms within the hemoglobin molecule and the integrity of the red blood cell membrane [3]. Iron can be either diamagnetic or paramagnetic, depending on the state of its outer electron orbitals. In the paramagnetic state, it alters the T1 and T2 relaxation times of water protons through magnetic dipole–dipole interactions and susceptibility effects. Dipole– dipole interactions shorten both the T1 and T2 relaxation times but have a greater effect on T1. Susceptibility effect is present when iron atoms are compartmentalized within the red cell membrane, causing magnetic field inhomogeneity, with resulting loss of phase coherence and selective shortening of the T2 relaxation time. After degradation of red cell membranes, the iron becomes more homogenously distributed, and this effect is nullified. Other factors that influence signal characteristics to a lesser extent include protein content, brain edema, oxygen tension, blood–brain barrier breakdown, thrombus formation, and clot retraction [2]. Both the chemical environment surrounding the hemoglobin iron atom and red cell membrane integrity undergo relatively predictable changes after ICH. The following section enumerates these changes in MR signal characteristics during the different phases of ICH evolution (Fig. 10 and Table 1).
- 10. Figure 10. MR imaging appearance of hemorrhage on T1-weighted (left column) and T2-weighted (right column) sequences for the different stages of hematoma (rows). Examples are selected from various patients. Hyperacute: There is relative isointensity on the T1-weighted and hyperintensity on the T2-weighted sequence of this right occipital hematoma. A small degree of vasogenic edema surrounds the hematoma. On the T2-weighted sequence there is a thin rim of hypointensity that is barely detectable in the periphery; this is caused by susceptibility effect from deoxy-hemoglobin. Acute: The marked hypointensity on the T2-weighted sequence of this left frontal hematoma is caused by susceptibility effect from deoxy-hemoglobin. Early subacute: The hyperintensity on the T1- weighted sequence of this right occipital hematoma is caused by the oxidation of deoxy-hemoglobin to met- hemoglobin. Late subacute: The hyperintensity on the T2-weighted sequence of this large left frontal hematoma results from the loss of susceptibility effect caused by degradation of the red cell membranes. The degree of vasogenic edema is lesser compared with earlier phases. Chronic: A former large right frontal hematoma has resolved into a slit-like cavity with a rim of hypointensity on the T2-weighted sequence caused by hemosiderin deposition. Table 2. Evolution of MR imaging signal characteristics with time
- 11. Iron Iron-containing Red cell Phase Time oxidation T1 T2 T2* molecule membranes state Hyperacute Hours Oxyhemoglobin Fe 2+ Intact Hypointense Hyperintense Acute Hours to Deoxyhemoglobin Fe 2+ Intact iso/ Hypointense Hypointense days Hypointense Early Days to 1 Methemoglobin Fe 2+ Intact Hyperintense Hypointense subacute week Late 1 week to Methemoglobin Fe 2+ Degraded Hyperintense Hyperintense subacute months Chronic >months Hemosiderin Fe 2+ Degraded iso/ Hypointense Hypointense Hypointense Abbreviations: Fe, iron; iso, isointense relative to normal brain.. Effect of hematoma evolution on MR imaging appearance of intracerebral hemorrhage Hyperacute phase The hyperacute phase of the hematoma is seen immediately following extravasation of blood into the brain parenchyma. At this stage the red cell membrane is intact, and the hemoglobin molecule is normally saturated with oxygen (oxy-hemoglobin). Specifically, the iron atoms contained within the heme portions of the hemoglobin molecule are bound to oxygen. This is the only phase of hematoma in which the iron atoms have no unpaired electrons in their outer orbitals and are therefore “diamagnetic,” without exaggerated T1 relaxation or susceptibility effects. The ICH signal characteristics are thus not primarily attributable to iron but instead to the increased spin density of the hematoma relative to uninvolved brain tissue. Hyperacute hematoma appears slightly hypointense or iso-intense on T1-weighted images and slightly hyperintense on T2-weighted images (see Fig. 10); this pattern resembles that of many other pathologic conditions of the brain. Even early in the hyperacute phase, however, there is often deoxy-hemoglobin at the periphery of the hematoma, which appears as a thin rim of T2 hypointensity. This pattern can help differentiate hyperacute hematoma from other brain pathologies [4–5]. Acute phase The acute phase, which begins within hours of ICH, is characterized by deoxy-hemoglobin. Deoxygenation occurs first at the periphery of the hematoma and progresses toward the center. This pattern appears because intrahematoma oxygen tension is lowest in the periphery, where red cells are adjacent to oxygen-starved tissue, and highest in the center, because red cells do not use oxygen for their metabolism. The iron atoms of deoxy-hemoglobin have five ligands and four unpaired electrons and hence are paramagnetic. Susceptibility effect is present because the iron is compartmentalized within intact red cell membranes, resulting in hypointensity on T2-weighted images that is due to increased T2* relaxation (see Fig. 10). Magnetic dipole–dipole interactions are prevented by the three- dimensional atomic structure of deoxy-hemoglobin, which blocks access of water protons to iron atoms. T1 relaxation times are therefore not shortened, and there is iso- or slight hypointensity on T1-weighted images (see Fig. 10). Sometimes a thin rim of T1 hyperintensity can be seen in the periphery of the hematoma, caused by early oxidation of intracellular deoxy-hemoglobin to intracellular met-hemoglobin. Early subacute phase After several days, the early subacute phase begins. The production of reducing substances declines with failure of red cell metabolism, and the iron atoms are oxidized to the ferric state, Fe3+, to produce met-hemoglobin. Magnetic dipole–dipole interactions can occur because the three-dimensional structure of met-hemoglobin exposes the iron atoms to water protons. This pattern leads to decreased T1 relaxation times and marked hyperintensity on T1- weighted images. Susceptibility effect is present because the red cell membranes remain intact, and hence there is continued hypointensity on T2-weighted images (see Fig. 10). Late subacute phase Over several days to weeks, the red cell membranes are degraded, and the late subacute phase begins. Susceptibility effect is lost because met-hemoglobin is no longer locally sequestered within red cell membranes; it freely diffuses within the hematoma cavity, resulting in a locally homogeneous magnetic field. This pattern leads to T2*
- 12. lengthening, and hence to increased hyperintensity, on T2-weighted images (see Fig. 10). Chronic phase Over the ensuing months, the hematoma enters the chronic phase. The degree of hyperintensity on T1- and T2- weighted images lessens as the concentration of met-hemoglobin decreases with protein breakdown. The center of the hematoma may evolve into a fluid-filled cavity with signal characteristics identical to cerebrospinal fluid, or the walls of the cavity may collapse, leaving only a thin slit (see Fig. 10). As proteins are degraded, the iron atoms become liberated from the heme molecules, scavenged by macrophages, and converted into ferritin, which can be recycled. In most cases, however, the degree of iron deposition overwhelms the recycling capacity, with the excess being locally concentrated in hemosiderin molecules. The iron in hemosiderin does not have access to water protons and therefore exerts only susceptibility effect without significant dipole–dipole interactions, leading to marked hypointensity on T2-weighted images. This hypointensity is seen at the rim of the hematoma cavity and may persist indefinitely. In practice, there is considerable variability in the orderly progression of hematoma signal change over time. The evolution of these signal characteristics may be influenced by a number of factors, including ICH size, oxygen tension, integrity of the blood–brain barrier, the presence of rebleeding, the efficiency of the patient's intrinsic repair processes, and the presence of an underlying lesion such as an arteriovenous malformation or tumor [2]. It is common to see different stages appear simultaneously. For these reasons, “dating” of bleed onset using MR imaging data alone is intrinsically imprecise. SUMMARY SUMMARY Hyperacute Hyperacute hemorrhage refers to a collection of blood in which oxyhemoglobin predominates. Hemorrhage (0 This type of hemorrhage is rarely encountered in the clinical setting because several hours TO 12 Hours) commonly elapse between the onset of hemorrhage and a patient being referred for MR imaging. A hyperacute hematoma may have a nonspecific appearance on MR imaging because oxyhemoglobin is not paramagnetic. Hyperacute hemorrhages can behave similar to other lesions with increased water content and are typically isointense or low signal on Tl-weighted images and high signal on T2- weighted images.With clot retraction in the hyperacute phase, the increased protein content of the hemorrhage results in the lesion being isointense to hyperintense on Tl- weighted images, which can facilitate differentiation from other mass lesions.A hypointense rim along the periphery of a hyperacute hemorrhage was noted ,and this may provide an additional clue to the diagnosis. Hyperacute hemorrhage, however, is more readily identified on CT, which remains the initial examination of choice for its evaluation. Acute In the acute setting, there is clot retraction and resorption of serum. As the hematoma Hemorrhage (12 becomes isolated from the normal cerebral circulation, oxyhemoglobin rapidly deoxygenates Hours to 3 Days) to yield deoxyhemoglobin. The increased protein content of the retracted clot, which is responsible for the high attenuation noted on noncontrast CT, causes the hematoma to be slightly hyperintense relative to low-intensity cerebrospinal fluid (CSF) and slightly hypointense to brain parenchyma on Tl-weighted images. In contrast to CT in the acute setting, which reliably demonstrates high attenuation characteristic of acute hemorrhage, MR imaging findings on Tl-weighted images are relatively nonspecific. T2-weighted images demonstrate a marked decrease in signal intensity because of magnetic susceptibility effects. The signal loss is due to local field inhomogeneity related to paramagnetic deoxyhemoglobin within intact red blood cells.Gradient echo images exaggerate this effect and are often useful for the diagnosis of acute hemorrhage Subacute In the early subacute stage, oxidation of deoxyhemoglobin to methemoglobin occurs within Hemorrhage: intact red blood cells. Methemoglobin is paramagnetic, which results in marked increased Early (3 Days to I signal intensity on Tl-weighted images.Typically the increased signal begins at the periphery of
- 13. Week) the clot and progresses inward. The differential diagnosis of lesions that demonstrate increased signal intensity on noncontrast TI-weighted images is relatively limited. Fat; substances with elevated protein content (mucus or fluid within certain intracranial tumors, i.e., craniopharyngioma); and paramagnetic moieties, such as melanin, free radicals (within the wall of parenchymal abscesses), and ions including calcium, manganese, and copper, can exhibit high signal intensity on Tl-weighted images. Correlation with the clinical history, morphology, and location of the lesion as well as appearance on other imaging sequences (i.e., T2, gradient echo, and fat suppression) typically allows differentiation from subacute hemorrhage. On T2-weighted images, paramagnetic methemoglobin in intact red blood cells results in low signal intensity similar to that of deoxyhemoglobin. The combination of bright signal intensity on Tl and markedly decreased signal intensity on T2 is relatively specific for paramagnetic substances and, with the appropriate morphology, is highly suggestive of intracranial hemorrhage Subacute The late subacute stage is characterized by lysis of the blood cells. Concurrently, there is Hemorrhage: Late dilution of extracellular methemoglobin and breakdown of the proteinaceous clot. High signal (1 Week to intensity persists on TI-weighted images because of methemoglobin; however, lysis of the red Months) blood cells and the decrease in protein content result in increased signal intensity on T2- weighted images.Bright signal intensity on both Tl-weighted and T2- weighted images is highly specific for hemorrhage . Furthermore, at the periphery of the hemorrhage, early accumulation of hemosiderin and ferritin within macrophages causes a low signal intensity ring, most prominent on T2-weighted images. Chronic stage The hallmark of chronic hemorrhage is low signal intensity on T2-weighted images because of (Months to Years) ferritin and hemosiderin within macrophages, which are the final breakdown products of hemoglobin. The resulting low intensity first appears during the late subacute stage of hematoma evolution at the margin of the lesion. With time, the rim thickens. A collapsed cavity with peripheral areas of low signal intensity on T2-weighted images is the residuum of an uncomplicated intraparenchymal hemorrhage. This appearance can persist indefinitely . Addendum A new version of this software is uploaded in my web site every week (every Saturday and remains available till Friday.) To download the current version follow the link quot;http://neurology.yassermetwally.com/crow.zipquot;. You can also download the current version from within the publication or go to my web site at quot;http://yassermetwally.comquot; to download it. At the end of each year, all the publications are compiled on a single CD-ROM, please contact the author to know more details. Screen resolution is better set at 1024*768 pixel screen area for optimum display REFERENCES References 1. Bradley WG. MR appearance of hemorrhage in the brain. Radiology. 1993;189(1):15–26. 2. Atlas SW, Thulborn KR. Intracranial hemorrhage. In: Atlas SW editors. Magnetic resonance imaging of the brain and spine. 3rd edition. Philadelphia: Lippincott Williams & Wilkins; 2002;. 3. Gomori JM, Grossman RI, Goldberg HI, et al.. Intracranial hematomas: imaging by high-field MR. Radiology. 1985;157(1):87–93. 4. Linfante I, Llinas RH, Caplan LR, et al.. MRI features of intracerebral hemorrhage within 2 hours from symptom onset. Stroke. 1999;30(11):2263–2267.
- 14. 5. Wintermark M, Maeder P, Reichhart M, et al.. MR pattern of hyperacute cerebral hemorrhage. J Magn Reson Imaging. 2002;15(6):705–709. 6. New PF, Aronow S. Attenuation measurements of whole blood and blood fractions in computed tomography. Radiology 1976;121:635-40. 7. Atlas SW, Thulbom KR. MR detection of hyperacute parenchymal hemorrhage of the brain. Am J Neuroradiol 1998;19:1471-507. 8. Gomori JM, Grossman RI, Goldberg HI, et al. Intracranial hematomas: imaging by high-field MR. Radiology 1985;157:87-93. 9. Wilberger JE, Rothfus WE, Tabas J, et al. Acute tissue tear hemorrhages of the brain: computed tomography and clinicopathological correlations. Neurosurgery 1990;27:208-13. 10. Barnett HJM, Yatsu FM, Mohr JP, Stein BM, eds.: Stroke: Pathophysiology, Diagnosis, and Management. 3rd ed. Churchill Livingstone; 1998. 11. Bradley WG Jr: MR appearance of hemorrhage in the brain. Radiology 1993 Oct; 189(1): 15-26. 12. Broderick JP, Brott T, Tomsick T: Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg 1993 Feb; 78(2): 188-91. 13. roderick JP, Brott TG, Duldner JE: Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993 Jul; 24(7): 987-93. 14. Challa VR, Moody DM, Bell MA: The Charcot-Bouchard aneurysm controversy: impact of a new histologic technique. J Neuropathol Exp Neurol 1992 May; 51(3): 264-71. 15. Chan S, Kartha K, Yoon SS: Multifocal hypointense cerebral lesions on gradient-echo MR are associated with chronic hypertension. AJNR Am J Neuroradiol 1996 Nov-Dec; 17(10): 1821-7. 16. Fazekas F, Kleinert R, Roob G: Histopathologic analysis of foci of signal loss on gradient-echo T2*- weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999 Apr; 20(4): 637-42. 17. Gokaslan ZL, Narayan RK: Intracranial Hemorrhage in the Hypertensive Patient. Neuroimaging Clinics of North America 1992; 2: 171-86. 18. Gomori JM, Grossman RI: Mechanisms responsible for the MR appearance and evolution of intracranial hemorrhage. Radiographics 1988 May; 8(3): 427-40. 19. Nelson JS, Parisi JE, Schochet SS Jr: Principles and Practise of Neuropathology. Mosby - Year Book, Inc. St. Louis, MO; 1993. 20. Robertson CS, Contant CF, Gokaslan ZL: Cerebral blood flow, arteriovenous oxygen difference, and outcome in head injured patients. J Neurol Neurosurg Psychiatry 1992 Jul; 55(7): 594-603. 21. Ruscalleda J, Peiro A: Prognostic factors in intraparenchymatous hematoma with ventricular hemorrhage. Neuroradiology 1986; 28(1): 34-7. 22. Spangler KM, Challa VR, Moody DM: Arteriolar tortuosity of the white matter in aging and hypertension. A microradiographic study. J Neuropathol Exp Neurol 1994 Jan; 53(1): 22-6. 23. Taveras JM, Pile-Spellman J: Neuroradiology. 3rd ed. Williams & Wilkins; 1996. 24. Welch KMA, Caplan LR, Reis DJ, Weir B, Siesjo BK, eds.: Primer on Cerebrovascular Diseases. Morgan Kaufmann; 1997.
- 15. 25. Metwally, MYM: Textbook of neurimaging, A CD-ROM publication, (Metwally, MYM editor) WEB-CD agency for electronic publication, version 8.4a october 2007
