Transition metals
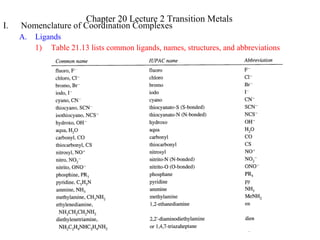
- 1. I. Nomenclature of Coordination Complexes A. Ligands 1) Table 21.13 lists common ligands, names, structures, and abbreviations Chapter 20 Lecture 2 Transition Metals
- 2. B. Naming and Writing Formulas of Coordination Compounds 1) The cation comes first, then the anion(s) a) diamminesilver(I) chloride [Ag(NH3)2]Cl b) potassium hexacyanoferrate(III) K3[Fe(CN)6] 2) Inner Sphere Complex Ion is enclosed in brackets a) Ligands are named before the metal b) Metal is written first in the formula c) Metal oxidation state in Roman Numerals in parenthesis after the metal ion d) A space only between cation and anion e) No capitalization is needed i. tetraamminecopper(II) sulfate [Cu(NH3)4]SO4 ii. hexaamminecobalt(III) chloride [Co(NH3)6]Cl3 3) Prefixes denote the number of each ligand type. Special prefixes and parentheses are used if the ligand already contains a prefix. 2 di bis 6 hexa hexakis 3 tri tris 7 hepta heptakis 4 tetra tetrakis 8 octa octakis 5 penta pentakis 9 nona nonakis 10 deca decakis
- 3. a) dichlorobis(ethylenediamine)cobalt(III) fluoride [Co(en)2Cl2]F b) tris(bipyridine)iron(II) chloride [Fe(bipy)3]Cl2 4) Ligands are named in alphabetical order not counting prefixes. a) tetraamminedichlorocobalt(III) [Co(NH3)4Cl2]+ b) amminebromochloromethylamineplatinum(II) [Pt(NH3)BrCl(CH3NH2)] 5) Ligand name alterations: a) Anionic ligands are given an -o suffix: chloro, flouro, oxo, sulfato b) Neutral ligands keep their name: methylamine, bipyridine c) Water becomes aqua d) NH3 becomes ammine to keep separate from alkylamines 6) How to handle anionic complexes a) Add –ate to the metal name if the complex ion has an overall (-) charge b) Negatively charged complexes of certain metals use their Latin names: Fe = ferrate Ag = argenate Sb = stibate Pb = plumbate Sn = stannate Au = aurate c) [PtCl4]2- = tetrachloroplatinate(II)
- 4. II. Coordination Chemistry Isomers = same ligands arranged differently A. Hierarchy of isomers
- 5. B. Structural Isomers = different ligands in coordination sphere 1) Coordination Isomers = ratio of ligand:metal same, but ligands are attached to metal ions in different numbers a) [Pt(NH3)2Cl2] b) [Pt(NH3)3Cl][Pt(NH3)Cl3] c) [Pt(NH3)4][PtCl4] 2) Linkage Isomers = depends on which atom of the ligand is attached to metal a) SCN- Linkage isomers i. Pb2+ —SCN = thiocyanate complex ii. Fe3+ —NCS = isothiocyanate complex b) NO2 - Linkage isomers i. M—ONO = nitrito complex ii. M—NO2 = nitro complex
- 6. C. Stereoisomers = same ligands, but different spatial arrangement 1) Geometric Isomers a) cis- or trans- isomers possible for MA2B2 b) Six-coordinate complexes also can have cis and trans isomers 2) Optical Isomers = have opposite effect on plane polarized light C. Stereoisomers = same ligands, but different spatial arrangement 1) Geometric Isomers a) cis- or trans- isomers possible for MA2B2 b) Six-coordinate complexes also can have cis and trans isomers 2) Optical Isomers = have opposite effect on plane polarized light
- 7. a. Optical Isomers are non-superimposable mirror images of each other b. Optical Isomers are called Enantiomers (Many biomolecules/drugs) c. An object or molecule that has an Enantiomer is called Chiral
- 8. III. Coordination Compounds and the Localized Electron Model A. History 1) Proposed by Pauling in the 1930’s 2) Describes bonding using hybrid orbitals filled with e- pairs 3) Extension of Lewis/VSEPR to include d-orbitals B. Theory 1) Metal ions utilize d-orbitals in hybrids 2) Octahedral complexes require 6 hybrid orbitals a) d2 sp3 hybridization of metal Atomic Orbitals provides new MO b) Ligand lone pairs fill the hybrid orbitals to produce the bond c) d-orbitals can come from 3d (low spin) or 4d (high spin) Fe3+ Co2+
- 9. 3) Coordinate Covalent Bond = Ligand as Lewis Base and Metal as Lewis Acid
- 10. 3) Problems with the theory a) High energy 4d orbitals are unlikely participants in bonding b) Doesn’t explain electronic spectra of transition metal complexes III. Crystal Field Theory A. History 1) Developed to describe metal ions in solid state crystals only 2) M+ is surrounded by A- “point charges” 3) Energies of the d-orbitals are “split” due to unequal geometric interactions with the point charges 4) Does not take into account covalency and molecular orbitals 5) Has been extended to do so in Ligand Field Theory B. Theory 1) Place degenerate set of 5 d-orbitals into an octahedral field of (-) charges (L:) 2) The electrons in the d-orbitals are repelled by the (-) charge of the ligands 3) The dz2 and dx2-y2orbitals are most effected because their lobes point directly along x,y,z axes where the point charges are 4) The dxy, dxz, and dyz orbitals aren’t destabilized as much
- 11. Octahedral Arrangement of d-Orbitals
- 12. 5) The energy difference between these orbital sets is called “delta octahedral” = ∆o a) The low energy set has t2g symmetry and are stabilized by –0.4 ∆o each b) The high energy set has eg symmetry and are destabilized by +0.6 ∆o each c) The total energy of the 5 d-orbitals is the same as in the uniform field = 0 (2)(+0.6 ∆o) + (3)(-0.4 ∆o) = 0
- 13. 6) CFSE = Crystal Field Stabilization Energy = how much energy is gained by the electrons in the 5 d-orbitals due to their splitting a) Co(III) = d6 low spin (6e-)(-0.4 ∆o) = -2.4 ∆o stabilization a) Cu(II) = d9 (6e-)(-0.4 ∆o) + (3e-)(+0.6 ∆o) = -0.6 ∆o stabilization a) Cu(I) = d10 (6e-)(-0.4 ∆o) + (4e-)(+0.6 ∆o) = 0 ∆o stabilization
- 14. MO Diagram for an Octahedral Complex
- 15. 7) All octahedral metal complexes will have the exact same MO diagram, only the number of d-electrons will change 8) The 6 bonding MO’s, with lowered energy for their electron pairs is what holds the metal complex together 9) The d-electrons in the t2g and eg* MO’s a) Determine the “Ligand Field” b) Determine the geometry and many characteristics of the metal complex C. Orbital Splitting and Electron Spin 1) The energy difference between the t2g and eg* MO’s = ∆o = “delta octahedral” 2) Strong-Field Ligands = ligands whose orbitals interact strongly with metal ion a) eg* is raised in energy b) ∆o is large 3) Weak-Field Ligands = ligands whose orbitals interact weakly with metal ion a) eg* is raised only slightly in energy b) ∆o is small
- 16. 4) Electron Spin a) d0 – d3 and d8 – d10 octahedral complexes have only one possible arrangement of electrons in the t2g and eg* MO’s b) d4 – d7 octahedral complexes have two possible electronic arrangements i. Low Spin = least number of unpaired electrons; favored by strong field ligands with large ∆o ii. High Spin = maximum number of unpaired electrons; favored by weak field ligands with small ∆o
- 17. 4) Splittings for other geometries: Tetrahedral Square Planar Linear
- 18. E. The Spectrochemical Series 1) A list of Strong-Field through Weak-Field ligands 2) σ-donors only a) en > NH3 because it is more basic (stronger field ligand) b) F- > Cl- > Br- > I- (basicity) 3) π-donors a) Halides field strength is lowered due to π-donor ability b) For similar reasons H2O, OH-, RCO2 - also are weak field ligands 4) π-acceptors increase ligand field strength: CO, CN- > phen > NO2- > NCS- 5) Combined Spectrochemical Series CO, CN- > phen > NO2- > en > NH3 > NCS- > H2O > F- > RCO2- > OH- > Cl- > Br- > I- Strong field, low spin π-acceptor σ-donor only Weak field, high spin π-donor
- 19. D. Electronic Spectra 1) A characteristic of transition metal complexes is color arising from electronic transitions between d-orbitals of different energies a) Electronic transition in an octahedral d1 complex b) The UV-Vis Experiment and the spectral result
