Face.ppt
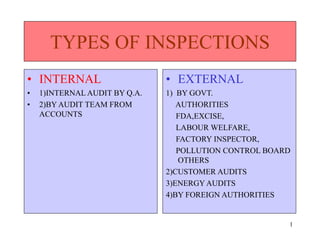
- 1. 1 TYPES OF INSPECTIONS • INTERNAL • 1)INTERNAL AUDIT BY Q.A. • 2)BY AUDIT TEAM FROM ACCOUNTS • EXTERNAL 1) BY GOVT. AUTHORITIES FDA,EXCISE, LABOUR WELFARE, FACTORY INSPECTOR, POLLUTION CONTROL BOARD OTHERS 2)CUSTOMER AUDITS 3)ENERGY AUDITS 4)BY FOREIGN AUTHORITIES
- 2. 2 Quality system approach to GMP Inspection 1. Pre – Approval Inspection (PAI) 2. CGMP Inspections are carried out Biennial (every tow years) Targets of this Inspections are Manufacturers & Policies. Objectives are as follows: Assure correlation between Manufacturing process for : a) Clinical trial b) Bioavailability study c) Stability studies d) Submission information
- 3. 3 Quality system approach to GMP Inspection 3. “For cause” Inspection, e.g. Field Alert filing, other health risks brought to Regulatory attention.
- 4. 4 System Based CGMP Inspection System based CGMP Inspection involves: 1.Quality System 2. Facility 3. Equipment 4.Utility 5. Materials 6. Production 7. Laboratory Controls 8. Packaging & Labeling If one of the eight systems is out of control, the company is considered out-of-control
- 5. 5 1.Quality System & Management Approach: 1. Assure overall compliance with CGMP regulations and all quality systems. 2. Quality must be built into the process & Quality is not tested into the product 3. Quality Assurance review Quality System and trending & involved in continuous improvement within well characterized process. 4. It should assures overall compliance with CGMPs.
- 6. 6 5.Quality Unit Reviews and approves following, a) Annual Product Quality Reviews b) Complaints c) Deviations/Failure Investigations d) Change Control e) CAPA f) Reprocess/Rework g) Validation/Revalidation h) Rejects i) Stability Failures/Out of Trend data j) Quarantine Products k) Documented Training – GMP and Job Related
- 7. 7 2. Facilities: 1. Location, design and construction of facility should be appropriate , to facilitate operations, maintenance and cleaning. 2. Layout and air – handling are designed and constructed in such a way to prevent cross- contamination 3. Flow of materials and personnel - designed to prevent mix- ps or contamination such as incoming Materials, Sampling Area, Quarantine Area, Released Area, Rejected material. 4. Separate facilities for hormones and highly potent compounds should be provided.
- 8. 8 3.Equipment: 1. Equipment should be of appropriate design, size, location and non-reactive product contact surfaces. 2. It should be clearly marked by code number for it’s identification. 3. Before installation it’s Qualification (DQ,IQ,OP,PQ) and calibration should be performed. 4. Maintenance department should prepare Preventive Maintenance Schedule and procedures.
- 9. 9 Equipment 5. Equipment cleaning procedures and cleaning validation should be performed to prevent contamination and mix-ups 6. Equipment Usage Log: Cleaning and maintenance should; be maintained. 7. Lubricant ,heating fluids or coolant should not alter product quality. 8. Equipment should be closed & Inspection should be done prior to use.
- 10. 10 4. Utility • Utility system involves following utilities: 1. HVAC system 2. Water system 3. Piping system 4. Drawing as Built
- 11. 11 UTILITY • Other Utilities: 1. Electricity 2. ETP system 3. Compressed Air system 4. Dust Extraction system
- 12. 12 UTILITY • All above system should be Qualified, validated, approved & appropriately monitored • Water used for sterile dosage form should be monitored and controlled for total microbial counts, objectionable organizations and endotoxins
- 13. 13 5. Materials System: 1. Material can include APIs, raw materials, process water,gas etc. 2. Material should be taken only from approved Vendor. 3. Periodic audit of supplier should be performed. 4. Change Control should be filled for changing suppliers and suppliers’ processes 5. Process Flow and written Procedures –Receipt, Identification, Quarantine, Storage, Handling, Sampling, Testing and approval/rejection of material should be according to CGMP.
- 14. 14 6.Production System: 1. Production and process controls should ensure safety, identity, strength, purity, quality of finished products. 2. Product should be validated & consistently produced. 3. Data should be documented and available to Quality Unit review – trending, investigation etc. 4. GMP & job related training should be given to all employees. 5. MFR, BMR, BPR, Change Control Procedure, In- process Control charts, tests, examinations,equipment Logs- Cleaning, Calibration, Qualifications, SOP’s etc. document should be well maintained & updated.
- 15. 15 7.Laboratory Control System: 1. Laboratory Procedures should be in place to ensure the accuracy of test results. 2. Quality Control Unit should be independent of production & should have adequate lab facilities, staff & supervisory bench personnel. 3. Written specifications – raw materials, APIs, intermediates, labels, packaging & finished products should be available in lab. 4. Written procedures –sampling, testing, approval or rejection of materials, recording and storage of data should be there. 5. Change control approval should be taken before any change.
- 16. 16 Laboratory Control System: 6. Reference standard – primary, secondary should be available in the lab. 7. Equipment Qualification, calibration & maintenance should be documented properly. 8. OOS Procedures for the timely investigation, documentation and conclusion of OOS investigation should be scientifically sound and justified. 9. Test Results of raw material, inprocess & finish product should be documented & Signed by person who perform it.
- 17. 17 1. Process Flows & written Procedures receipt, identification, quarantine, sampling, testing and approval/rejection of packaging and labeling materials should be maintained. 2. Label Storage Area – Should have enclosed (locked), limited access. 3. Reconciliation should be properly documented & investigation should be done if any discrepancy is observed. 8.Packaging & Labeling System:
- 18. 18 4. All excess labels coded with batch number, to be destroyed and documented 5. Print labels checked against master and a copy placed with the batch record. 6. Packaging operations should be designed to prevent mix-ups. 8.Packaging & Labeling System:
- 19. 19 Management Responsibilities A) Management Responsibilities involves: 1. Document Organogram of employee to ensure the assigned authorities and responsibilities. It should be including Name, Revision No., Effective date. 2. Management should support the total quality model . 3. The Quality Manager has the authority to detect problems ,implement solutions, and provide prompt feedback on quality issues to the organization. 4. It play a key role in the design, implementation, and management of robust quality systems
- 20. 20 5. Actively participate in Management Review meetings – to ensure continuing suitability, adequacy, and effectiveness 6. It advocate continuous improvement of the operation of Quality System. a) Strong and visible support for the quality system b Internal communication on quality issues at all levels 7. Management controls such as CAPA***,People qualifications & Training Records, Change control, Validation are always reviewed by Regulatory bodies, so it is responsibility of management to keep all these document updated. ` Management Responsibilities A) Management Responsibilities involves:
- 21. 21 B) Resources 1. It involves General Arrangements of Infrastructure, materials, products, testing 2. Personnel Development including Organizational culture, Qualifications and responsibilities 3. Management should supply and maintain the appropriate facilities and equipment 4. Control Outsourced Operations such as Quality Agreements& Qualifications and standards.
- 22. 22 5. QC is responsible for Analyze finished products and materials – collection, storage, examination of inprocess, stability, and reserve samples 6. HR department should be involved in new entrant training. It should be Problem solving and communicative in culture. They should define qualification and assign appropriate responsibilities such as Education, training and experience. 7. Manufacturing department should be involved in Design, develop and document Product and Processes by experts by analyzing critical processes. It is involved in Examining material & in performing and monitoring operations. It also address Nonconformities. 8. Evaluation Activities are done by conducting internal Audits (self Inspection), Analyzing Data for trends & CAPA B) Resources
- 23. 23 Most Common Observations of Regulatory Inspections: 1) No or inadequate procedures or procedures not followed 2) No management review of procedures 3) Management oversight / QA oversight 4) QC unit did not follow procedures 5) No internal audits performed 6) Inadequate employee training
- 24. 24 7) process not validated 8) Inadequate laboratory controls 9) Raw data not reviewed/maintained 10) No procedures for handling OOS situations 11) No procedures for Corrective and preventive actions 12) Recurring deviations Most Common Observations of Regulatory Inspections:
- 25. 25 How to minimize the risk of receiving non-compliance? 1. Know regulations and guidelines – the quality intent and meaning 2. Know the consequences of deviations of non – compliance – Both staff and management 3. Study 483s and Warning Letters from internet – Learn about mistakes others have made 4. Build up a quality system in line with industry standards, regulations and guidelines
- 26. 26 5. Develop SOP’s on Preparing for Inspections 6. Ongoing training to staff and management on GMP trends and new regulations 7. Respond to Regulatory observations effectively and timely. How to minimize the risk of receiving non-compliance?
- 27. 27 Conclusion 1. Implementation of a comprehensive, robust Quality system Model 2. Proactive approach to achieve long – term benefits 3. Characteristic of a Successful Quality System: a) Science Based Approaches b) Understand the intended use of a product c) Identify and control potential weak processes d) Timely remediation of deviation and investigation e) Sound methods for assessing and reducing risks f) Well defined processes and products – Product life Cycle g) Systems for careful analysis of product quality h) Supportive Management – philosophically and financially
- 28. 28 Thank You!
- 29. 29 How to Host Regulatory / Quality Audits Audit Sequence should be as follows: 1. Audit the analyst to describe the process. 2. Observe the process. 3. Read the SOP. 4. Check the records
- 30. 30 1. Ask how OOSs are investigated in QC and R&D Labs, how OOS is captured in LIMS 2. At Receiving Ask how samples are received into LIMS At Laboratory Ask how Lab Manager schedule samples for testing using LIMS At QA Ask how they approve samples and C of As using LIMS Style – Looking for Consistency Consistency among Different Department / Different Sites
- 31. 31 1. Company should Knows the GMP relates to their products or services 2. Company should knows why it has to do, in this way. 3. Company should provides documentation to show that the products are tested correctly, as per registration (NDA) 4. Company should have consistent approach in different departments Quality Auditor’s/ Inspector’s Expectation
- 32. 32 1. Understand GMP 2. Understand roles and responsibilities of self and direct reports 3. Demonstrates ability to justify, rationalize and defend at high level 4. Demonstrate control of the functional area. 5. Knowledge of area’s process and operational SOP’s 6. Knowledge of trends, failures, OOS, and recurring deviations 7. Knowledge of major projects, volumes, and capacities Expectation from Senior Managers
- 33. 33 1. He should understand the processes and products 2. GMP related activities, e.g. Equipment IQ,OQ,PQ program, validation status etc. 3. Able to present processes clearly – High Level 4. He/She should Know the quality trends, e.g. OOS, complaints, recurring issues, CAPA & Training Records. Expectation from Section Heads/Area Managers
- 34. 34 1. He/She should Know the processes, SOP’s and products – in detail 2. Present with clarity 3. Training records Expectation from Experts
- 35. 35 Expectations from Technical Staff Analyst/Specialist/Operator/Engineer/Project Leader should - 1. Demonstrate control of the tasks 2. Detailed knowledge and skills of SOP’s 3. Detailed knowledge and ‘hands-on’ experience in generation and recording of logbooks, and other supporting activities in your area.
- 36. 36 Investigator want to see PITCH What is PITCH? It is totality of, 1.Professionalism 2.Integrity 3.Technical Expertise 4.Communication Skills 5.Honesty
- 37. 37 Professionalism What is professionalism? Professional means you should - 1. Be prepared – Has information, documents, and thought organized and ready 2. Speaks of what he/she knows, defers to others as necessary 3. Works and speaks efficiently 4. Conveys confidence in words and action 5. Concerned with quality results 6. Speaks about pertinent issues, not personalities or unrelated topics 7. Conveys pride in own functional areas and company
- 38. 38 Integrity What is Integrity? Integrity means you should - 1. provides information, professional opinions, or impressions that are based on actual events and documents 2. Appropriate represents facts, possibilities, and professional opinions 3. Corrects any potentially misunderstandings quickly and courteously 4. Is willing to take appropriate responsibility
- 39. 39 Technical Expertise Technical Expertise is a person who - 1. Mentally prepares the answer and presents it in a logical manner 2. Provide scientific rationale. 3. Provides facts, documents and professional opinions as requested 4. Arrive at a valid, technically defending conclusion by considering ALL data and information
- 40. 40 Communication – Vocal 1. Speaks understandably at an appropriate rate, pitch and volume 2. Uses words, phrases that are respectful, appropriate, and thoughtful; not judgmental or emotionally charged 3. Speech patterns are efficient; pauses are used for thoughts 4. Tone of voice is neutral or pleasant
- 41. 41 Communication – Non – Verbal 1. Body posture conveys interest, confidence and openness 2. Hands and gestures are used for emphasis 3. Eye contact conveys interest, confidence and helps with the “connection” 4. Nervousness is appropriately managed by deep breathing and preparation 5. Overall appearance is professional and appropriate
- 42. 42 Honesty 1. Admit mistakes 2. Willing to take appropriate responsibility
- 43. 43 Inspector’s Targets 1. The greatest patient risks come from: a) Unexpected Deviations b) Change Controls c) Errors(OOS, Product/Method Transfer) 2. Quality Management: a) CAPA b) Complaints c) Rejected Batches,Retests,Re-assays d) Personnel 3. Interfaces between departments and sites
- 44. 44 Easy Targets for Inspection 1. Analysts not correctly garmented 2 Identification stickers peeling off from equipment 3. Rooms & equipment with no status labels 4. Lack of security to the Label storage areas 5. Area that is cluttered 6. Illegible identification tags 7. Unsigned documents
- 45. 45 Checkpoints before Audit - 1. What is in your garbage bin? 2. Protection of your password 3. Cleanliness of area 4. Are you wearing the correct garments or not?
- 46. 46 Other Preparations Facilities 1. First Impression 2. Housekeeping 3. Visual factory/Posters/Pictures 4. Lab coats 5. Staff
- 47. 47 How to Interact with the Inspector? 1. Always tell the truth. 2. Provide information as rapidly as possible. 3. Understand the question before answering. 4. Keep your responses short, but up to the point. 5. Don’t talk and work at the same time, expect to demonstrate your work to the inspector
- 48. 48 How to Interact with the Inspector? 6. Answer questions professionally, courteously, and carefully 7. Answer with facts. 8. It’s acceptable to say that you don’t know. 9. Correct or clarify any misinformation provided to the inspector.
- 49. 49 Types of Questions asked by Inspector’s 1. Direct questions – “Do you have an SOP for ………………?” 2. Leading questions – “Why did/didn’t you …………………..?” 3. Open – ended questions “Tell me some more about that…………”
- 50. 50 How to respond to the Auditor? 1. Don’t take them personally 2. Respond a) Positively b) Professionally 3. Never lose your temper!
- 51. 51 Answering Questions 1. Person with direct knowledge of the answer. 2. Don’t provide a “hearsay” answer. 3. Don’t answer for another department, if not sure 4. Speak from personal knowledge 5. Use SOP to resolve uncertainty
- 52. 52 Style - The Long Silence Technique Only answer the question which is asked …………then keep quiet!
- 53. 53 Style – Intimidation Technique 1. Don’t be intimidated 2. Make sure that you understand the question & make sure the inspector understands your answer
- 54. 54 DO’s During the Inspection 1. If the inspector is ‘digging’ or asking the same basic question, provide more information as answer may not be complete. 2. Talk about what is and not what might be or should be. 3. It a problem has surfaced, point out where GMP and quality system controls have worked and how improvements have been made. 4. If an activity went well, tell why.
- 55. 55 Inspector’s Requests 1. Satisfy requests for documents quickly 2. Only provide what is requested – and no more …………..
- 56. 56 What Arouse Suspicions? 1. Data that is “too good” 2. SOPs all bear same revision date 3. SOPs not followed as written 4. Evasive answers to avoid commitment or answer indirectly
- 57. 57 What Arouse Suspicions? 5. Body language/ eye contact 6. Obvious confusion among staff 7. Stalling tactics – Taking too long to obtain documents – Asking for the same information many times and not getting it. 8. Hiding unpleasant deviations, OOSs’ etc
- 58. 58 What you will do ,When you know that there is a problem 1. Check the most recent OOS or deviation, and it should be properly completed the investigation and explain the situation. 2. Auditor’s doesn’t expect perfection – That’s why we have a QA system 3. What was done about the problem? (CA) 4. What is the plan for future actions? 5. How will you prevent recurrence? (PA) 6. Always tell the truth
- 59. 59 Managing the Difficult Encounters 1. Understand what the investigator is asking 2. Know the limits to your knowledge 3. Don't feel pressured or intimidated 4. Don’t fill the gaps with wrong information or personal opinions 5. Maintain professionalism and integrity 6. Signal QA Host for help
- 60. 60 Don’ts - During the Inspection 1. Don’t try to control the conversation 2. Don’t try to change the agenda 3. Don’t waste inspector’s time 4. Don’t try any distraction techniques 5. Don’t leave an inspector alone 6. Don’t volunteer information 7. Don’t try to buy them expensive dinner or gifts
- 61. 61 Confrontation Avoid confrontation YOU CAN NOT WIN
- 62. 62 Phrases to Avoid : ‘I think this is what happens …….” 1. Unacceptable if responsible 2. Do not create your answer 3. Admit your uncertainty 4. Find the right person
- 63. 63 Phrases to Avoid: ‘Well, normally …….’ 1. How about abnormal situations? 2. Have you documented and validated process for abnormal situations? 3. Only answer question asked
- 64. 64 Phrases to Avoid: ‘Its too expensive’ 1. Unacceptable 2. Suggest alternatives
- 65. 65 Phrases to Avoid: ‘Not my problem’ 1. Negative reaction 2. Trigger investigation of all aspects of control
- 66. 66 Phrases to Avoid: ‘We have changed the process.’ 1. Out of date SOP 2. Changes which impact on product quality 3. Lack of change control 4. SOPs must be current
- 67. 67 Don’t be afraid to say “no” Inspector: “I worked on one of those many years ago. That’s a nice looking HPLC. Do you mind if I just press some of these buttons?”
- 68. 68 More Examples of Bad Comments 1. Yes. Yes. Yes……. 2. I’ m glad you’re here this week instead if the last week. 3. This is not my job. I’ve just transferred to this department last week. 4. I think this is the way to do it. I’m not sure. 5. I was never trained on this process.
- 69. 69 More Examples of Bad Comments 6. I don’t know anything. I just work here. 7. No problem, honey. 8. I’ve told my manager/staff many times, but he ignores the problem. 9. I know this is the right process but it takes too long. 10.We don’t have time to log the info. We enter data at the end.
- 70. 70 Most Examples of Bad Comments 1. We are too busy right now. 2. This equipment is non-GMP. 3. We have passed the other regulatory inspections in the past. 4. I don’t get support from other department. 5. We have been doing this for years 6. We have a budget restraint to hire more people. 7. That should not have happened. My staff should not have done that.
- 71. 71 Before Meeting the Investigators 1. Clearly define your area of responsibility or knowledge. 2. Have a working knowledge of the products, policies, methods and SOPs 3. Know why you do certain things. What is the (written) rationale? 4. Think through possible questions 5. Review the documents for weakness and prepare to answer questions
- 72. 72 The Golden Rules 1. Be prepared and be confident 2. If you do not know, admit it 3. Wrong answer versus 5 minutes wait and get right answer 4. Promptly provide documents
- 73. 73 Respect: A Two Way Process 1. Remember the inspector is professional with an important and necessary job. 2. Respect the inspector’s professionalism and they are more likely to respect yours
- 74. 74 Key to success: People 1. Competent 2. Confident 3. Credible 4. Knowledgeable 5. Honest 6. Able to demonstrate the strength of your company
- 75. 75 ……….and finally………… 1. Be concise, courteous, direct and honest in your dealings with investigators and stay cool 2. Don’t argue, speculate or blame others for mistakes 3. Remember inspectors are there to protect consumers/ patients by making sure we are producing quality products and services 4. That’s our job too!
- 76. 76 Thank You !