Uv visible spectroscopy
- 1. UV-VISIBLE SPECTROSCOPY DR. BASAVARAJAIAH S. M. M. SC., PH.D. ASSISTANT PROFESSOR AND COORDINATOR PG DEPARTMENT OF CHEMISTRY VIJAYA COLLEGE BENGALURU-560 004
- 2. Contents: 1. Introduction 2. Instrumentation 3. Classification of electronic transitions 4. Substituent and solvent effects 5. Applications of UV Spectroscopy 6. UV spectral study of alkenes 7. UV spectral study of poylenes 8. UV spectral study of α, β-unsaturated carbonyl 9. UV spectral study of Aromatic compounds 10. Empirical rules for calculating λmax.
- 3. •The term "spectroscopy" defines a large number of techniques that use radiation to obtain information on the structure and properties of matter. •The basic principle shared by all spectroscopic techniques is to shine a beam of electromagnetic radiation onto a sample, and observe how it responds to such stimulus. •The response is usually recorded as a function of radiation wavelength. •A plot of the response as a function of wavelength is referred to as a spectrum. INTRODUCTION
- 4. THE ELECTROMAGNETIC SPECTRUM Important: As the wavelength gets shorter, the energy of the radiation increases.
- 5. 1. Electromagnetic radiation displays the properties of both particles and waves 2. The particle component is called a photon 3. The energy (E) component of a photon is proportional to the frequency . Where h is Planck’s constant and υ is the frequency in Hertz (cycles per second) E = hν 4. The term “photon” is implied to mean a small, massless particle that contains a small wave-packet of EM radiation/light.
- 6. Ultraviolet radiation stimulates molecular vibrations and electronic transitions. Absorption spectroscopy from 160 nm to 780 nm. Measurement absorption or transmittance. Identification of inorganic and organic species. UV-Vis Spectroscopy
- 7. UV/VIS SPECTROSCOPY Visible (380-780 nanometers). Ultraviolet (UV) (10 – 380 nanometers). Below 200 nm, air absorbs the UV light and instruments must be operated under a vacuum
- 8. Principle of UV-Visible Spectroscopy •UV spectroscopy is type of absorption spectroscopy in which light of ultra-violet region (200-400 nm) is absorbed by the molecule which results in the excitation of the electrons from the ground state to higher energy state. •Molecules containing π-electrons or non-bonding electrons (n- electrons) can absorb energy in the form of ultraviolet light to excite these electrons to higher anti-bonding molecular orbital's. •The absorption of ultraviolet light by a chemical compound will produce a distinct spectrum which aids in the identification of the compound.
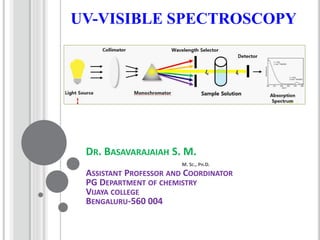
- 9. INSTRUMENTATION Light Source: •Tungsten filament lamps and Hydrogen-Deuterium lamps are most widely used and suitable light source as they cover the whole UV region. •Tungsten filament lamps are rich in red radiations; more specifically they emit the radiations of 375 nm, while the intensity of Hydrogen-Deuterium lamps falls below 375 nm. Note: Most of the spectrophotometers are double beam spectrophotometers.
- 10. Monochromator: •Monochromators generally is composed of prisms and slits. •The radiation emitted from the primary source is dispersed with the help of rotating prisms. •The various wavelengths of the light source which are separated by the prism are then selected by the slits such the rotation of the prism results in a series of continuously increasing wavelength to pass through the slits for recording purpose. •The beam selected by the slit is monochromatic and further divided into two beams with the help of another prism.
- 11. Sample and reference cells: •One of the two divided beams is passed through the sample solution and second beam is passed through the reference solution. •Both sample and reference solution are contained in the cells. •These cells are made of either silica or quartz. Glass can’t be used for the cells as it also absorbs light in the UV region. Detector: •Generally two photocells serve the purpose of detector in UV spectroscopy. •One of the photocell receives the beam from sample cell and second detector receives the beam from the reference. •The intensity of the radiation from the reference cell is stronger than the beam of sample cell. This results in the generation of pulsating or alternating currents in the photocells.
- 12. Amplifier: •The alternating current generated in the photocells is transferred to the amplifier. •The amplifier is coupled to a small servometer. •Generally current generated in the photocells is of very low intensity, the main purpose of amplifier is to amplify the signals many times so we can get clear and recordable signals. Recording devices: •Most of the time amplifier is coupled to a pen recorder which is connected to the computer. •Computer stores all the data generated and produces the spectrum of the desired compound.
- 13. Electronic transitions The absorption of UV or visible radiation corresponds to the excitation of outer electrons. There are three types of electronic transition which can be considered; •Transitions involving π, σ, and n electrons •Transitions involving charge-transfer electrons •Transitions involving d and f electrons (not covered in this Unit) CLASSIFICATION OF ELECTRONIC TRANSITIONS
- 14. •When an atom or molecule absorbs energy, electrons are promoted from their ground state to an excited state. •In a molecule, the atoms can rotate and vibrate with respect to each other. •These vibrations and rotations also have discrete energy levels, which can be considered as being packed on top of each electronic level.
- 15. Possible electronic transitions of π, σ, and n electrons are;
- 16. σ σ* Transitions: An electron in a bonding s orbital is excited to the corresponding antibonding orbital. The energy required is large. For example, methane (which has only C-H bonds, and can only undergo σσ* transitions) shows an absorbance maximum at 125 nm. Absorption maxima due to σσ* transitions are not seen in typical UV-Vis. spectra (200 - 700 nm). nσ* Transitions: Saturated compounds containing atoms with lone pairs (non-bonding electrons) are capable of nσ* transitions. These transitions usually need less energy than nσ* transitions. They can be initiated by light whose wavelength is in the range 150 - 250 nm. The number of organic functional groups with nσ* peaks in the UV region is small.
- 17. n π* and ππ* Transitions: Most absorption spectroscopy of organic compounds is based on transitions of n or π electrons to the π* excited state. This is because the absorption peaks for these transitions fall in an experimentally convenient region of the spectrum (200 - 700 nm). These transitions need an unsaturated group in the molecule to provide the p electrons. Note: Molar absorbtivities from n π* transitions are relatively low, and range from 10 to100 L mol-1 cm-1. ππ* transitions normally give molar absorbtivities between 1000 and 10,000 L mol-1 cm-1 .
- 18. SUBSTITUENT AND SOLVENT EFFECTS •The solvent in which the absorbing species is dissolved also has an effect on the spectrum of the species. •Peaks resulting from nπ* transitions are shifted to shorter wavelengths (blue shift) with increasing solvent polarity. •This arises from increased solvation of the lone pair, which lowers the energy of the n orbital. Often (but not always), the reverse (i.e. red shift) is seen for π π* transitions. •This is caused by attractive polarisation forces between the solvent and the absorber, which lowers the energy levels of both the excited and unexcited states. •This effect is greater for the excited state, and so the energy difference between the excited and unexcited states is slightly reduced-resulting in a small red shift. This effect also influences n π* transitions but is overshadowed by the blue shift resulting from solvation of lone pairs.
- 20. Auxochromes
- 22. 1.Bathochromic Shift or Red shift: A shift of an absorption maximum towards longer wavelength (λ) or lower energy (E). 2. Hypsochromic Shift or Blue Shift: A shift of an absorption maximum towards shorter wavelength (λ) or higher energy (E). 3.Hyperchromic Effect: An effect that results in increased absorption intensity (ε). 4.Hypochromic Effect: An effect that results in decreased absorption intensity (ε).
- 24. Applications of UV Spectroscopy Detection of Impurities •It is one of the best methods for determination of impurities in organic molecules. •Additional peaks can be observed due to impurities in the sample and it can be compared with that of standard raw material. •By also measuring the absorbance at specific wavelength, the impurities can be detected. Structure elucidation of organic compounds It is useful in the structure elucidation of organic molecules, such as in detecting the presence or absence of unsaturation, the presence of hetero atoms.
- 25. UV absorption spectroscopy can be used for the quantitative determination of compounds that absorb UV radiation. UV absorption spectroscopy can characterize those types of compounds which absorbs UV radiation thus used in qualitative determination of compounds. Identification is done by comparing the absorption spectrum with the spectra of known compounds. This technique is used to detect the presence or absence of functional group in the compound. Absence of a band at particular wavelength regarded as an evidence for absence of particular group. Kinetics of reaction can also be studied using UV spectroscopy. The UV radiation is passed through the reaction cell and the absorbance changes can be observed.
- 26. Many drugs are either in the form of raw material or in the form of formulation. They can be assayed by making a suitable solution of the drug in a solvent and measuring the absorbance at specific wavelength. Molecular weights of compounds can be measured spectrophotometrically by preparing the suitable derivatives of these compounds. UV spectrophotometer may be used as a detector for HPLC.
- 27. Again, demonstrates the moieties contributing to absorbance from 200-800 nm, because π electron functions and atoms having no bonding valence shell electron pairs. UV SPECTRAL STUDY OF ALKENES
- 28. Influence of conjugation on UV absorption
- 29. UV Spectra of 1, 3-Butadiene
- 30. UV Spectra of Isoprene
- 32. UV Spectra of Polyene •With increase in the number of double bonds in conjugation, the values of absorption maximum as well as intensity increase.
- 33. λmax = 455 nm λmax = 471 nm
- 34. UV spectra of Carbonyl Compounds
- 35. UV Spectra of Aromatic Compounds
- 36. UV Spectra of Naphthalene, Anthracene and Tetracene
- 37. Empirical Rules for Calculating λmax Woodward-Fieser Rules for Calculating λmax in Conjugated Dienes and Trienes Woodward (1941) formulated a set of empirical rules for calculating or predicting λmax in conjugated acyclic and six- membered ring dienes. These rules, modified by Fieser and Scott on the basis of wide experience with dienes and trienes, are called Woodward-Fieser rules and are summarized in next Table. First, we discuss the following terms used in Woodward-Fieser rules.
- 39. Homoannular Dienes •In homoannular dienes, conjugated double bonds are present in the same ring and having s-cis (cisoid) configuration (s = single bond joining the two doubly bonded carbon atoms). •The s-cis configuration causes strain which raises the ground state energy Ievel of the molecule leaving the high energy excited state relatively unchanged. Thus, the transition energy is lowered resulting in the shift of absorption position to a longer wavelength. In compounds containing both homoanular and heteroannular diene systems, the calculations are based on the longer wavelength (253 nm), i.e. the homoannular diene system.
- 40. Heteroannular Dienes: In heteroannular dienes, conjugated double bonds are not present in the same ring and these have s-trans (transoid) configurations: Exocyclic Conjugated Double Bonds: The carbon-carbon double bonds projecting outside a ring are called exocyclic double bonds. For example Note that the same double bond may be exocyclic to one ring, while endocyclic to the other and sometimes the same double bond may be exocyclic to two rings simultaneously.
- 41. Alkyl Substituents and Ring Residues: Only the alkyl substituents and ring residues attached to the carbon atoms constituting the conjugated system of the compound are taken into account. Following examples indicate such carbon atoms by numbers and the alkyl substituents and ring residues by dotted lines:
- 42. Examples:
- 44. Base value=253 (homoannular) 4 ring residues =4X5=20 2 exoyclic bond=2X5=10 Thus λmax = 283 nm Base value=214 (heteroannular) 4 ring residues =4X5=20 1 exoyclic bond=1X5=5 Thus λmax = 239 nm 1. Which of the following alkenes would have the largest λmax?
- 45. Fieser-Kuhn rule is used to calculate λmax for Polyenes According to the Fieser-Kuhn rule the following equation can be used to solve for the wavelength of maximum absorption λmax and also maximum absorptivity εmax: λmax = 114 + 5M + n (48.0 – 1.7 n) – 16.5 Rendo – 10 Rexo where, λmax is the wavelength of maximum absorption M is the number of alkyl substituents / ring residues in the conjugated system n is the number of conjugated double bonds Rendo is the number of rings with endocyclic double bonds in the conjugated system Rexo is the number of rings with exocyclic double bonds in the conjugated system. εmax = (1.74 x 104) n where, εmax is the maximum absorptivity n is the number of conjugated double bonds
- 46. Name of Compound β-Carotene Base Value 114 nm M (number of alkyl substituents) 10 n (number of conjugated double bonds) 11 Rendo (number of endocyclic double bonds) 2 Rexo (number of exocyclic double bonds) 0 Substituting in equation λmax = 114 + 5M + n (48.0 – 1.7 n) – 16.5 Rendo – 10 Rexo = 114 + 5(10) + 11 (48.0-1.7(11)) – 16.5 (2) – 10 (0)= 114 + 50 + 11 (29.3) – 33 – 0= 114 + 50 + 322.3 – 33 Calc. λmax = 453.30 nm λmax observed practically 452nm Calculate εmax using equation: εmax = (1.74 x 104) n = (1.74 x 104) 11Calc. εmax= 19.14 x 104 Practically observed εmax 15.2 x 104
- 47. Name of Compound all-trans-lycophene Base Value 114 nm M (number of alkyl substituents) 8 n (number of conjugated double bonds) 11 Rendo (number of endocyclic double bonds) 0 Rexo (number of exocyclic double bonds) 0 Substituting in equation λmax = 114 + 5M + n (48.0 – 1.7 n) – 16.5 Rendo – 10 Rexo = 114 + 5(8) + 11 (48.0-1.7(11)) – 16.5 (0) – 10 (0)= 114 + 40 + 11 (29.3) – 0 – 0= 114 + 40 + 322.3 – 0 Calc. λmax = 476.30 nm λmax observed practically 474nm Calculate εmax using equation: εmax = (1.74 x 104) n = (1.74 x 104) 11Calc. εmax= 19.14 x 104 Practically observed εmax 18.6 x 104
- 48. Name of Compound Retinol Base Value 114 nm M (number of alkyl substituents) 5 n (number of conjugated double bonds) 5 Rendo (number of endocyclic double bonds) 1 Rexo (number of exocyclic double bonds) 0 Substituting in equation λmax = 114 + 5M + n (48.0 – 1.7 n) – 16.5 Rendo – 10 Rexo = 114 + 5(5) + 5 (48.0-1.7(5)) – 16.5 (1) – 10 (0) = 114 + 25 + 5 (39.5) – 16.5 – 0 = 114 + 25 + 197.5 – 16.5 – 0 Calc. λmax = 320 nm λmax observed practically 325 nm Calculate εmax using equation: εmax = (1.74 x 104) n = (1.74 x 104) 5Calc. εmax= 8.7 x 104 Practically observed εmax N/A
- 49. Woodward-Fieser Rules for Calculating λmax in α, β Unsaturated Carbonyl Compounds •Compounds containing a carbonyl group (C=O) in conjugation with an ethylenic groups (C=C) are called enones. •UV spectra of enones are characterized by an intense absorption band (K-band) due to ππ* transition in the range 215-250 nm (εmax usually 10,000-20,000) and a weak R-band due to nπ * transition in 310-330 nm region (εmax usually 10-100). •Similar to dienes and trienes, there are set rules called Woodward- Fieser rules for calculating or predicting λmax in α, β -unsaturated carbonyl compounds (enones). •These rules first framed by Woodward and modified by Fieser and by Scott are given in Table.
- 51. Examples:
- 52. Base value=215 2 β-ring residues=2X12=24 1 exocyclic double bond =1X5=5 Calc. λmax = 244 Base value=202 1 α-substituent=1X10=10 1 β-alkyl substituents= 1X12=12 1 γ- ring residues =1X18=18 1 δ- ring residues = 1X18=18 1 double bond extending conjugation=1 X 30=30
- 53. Base value=215 1 β-alkyl substituents= 1X12=12 1 β-ring residues=1X12=12 1 α-OH substituent=1X35=35 Calc. λmax = 274 Base value=195 1 α-substituent=1X10=10 2 β-ring residues=2X12=24 1 exocyclic double bond =1X5=5 Calc. λmax = 234 nm
- 54. Woodward-Fieser Rule for benzene and its derivatives Like Woodward-Fieser rules, Scott formulated a set of rules for calculating the absorption maximum of the primary absorption band of aromatic aldehydes, ketones, carboxylic acids and esters. In the absence of steric hindrance to co-planarity, the calculated values are within + 5 nm of the observed value. 1. Base values for: a) ArCOR = 246 nm b) ArCHO = 250 nm c) ArCO2H = 230 nm d) ArCO2R = 230 nm
- 55. 2. Increment for substituents: Substituents Ortho In nm Meta In nm Para In nm Alkyl group or ring residue 3 nm 3 nm 10 nm –OH, –OCH3, –OAlkyl 7 7 25 –O (oxonium) 11 20 78 –Cl 0 0 10 –Br 2 2 15 –NH2 13 13 58 –NHCOCH3 20 20 45 -NHCH3 - - 73 -N(CH3 )2 20 20 85
- 56. Base value=246 2 m-OH=2 x 7=14 1 p-OH=1 x 25=25 Calc. λmax = 285 nm Base value=246 Ring residue at ortho position =1 x 3=3 1 p-OCH3=1 x 25=25 Calc. λmax = 274 nm Base value=246 2 o-O Alkyl=2 x 7=14 1 m-Cl=1 x 0=0 1 p-OCH3=1 25=25 Calc. λmax = 285 nm
- 57. 1. Calculate the λ max for the following molecules O O CH3 CH3 O Cl CH3 CH3 O 1. 2. 3. For Practice:
- 58. 2. Which molecule absorbs at the longest wavelength, 1,3-hexadiene or 1,4-hexadiene? 3. Why the λmax for the diene (I) is observed at lower nm than (II). (I) (II)
- 59. 4. What are the products of these reactions? Would you expect them to have higher or lower λmax than the starting material?
- 60. Spectroscopy Learning Websites 1. http://www.rsc.org/learn- chemistry/collections/spectroscopy. 2. http://www.rsc.org/learn- chemistry/resource/res00001041/spectroscopy-videos. 3. http://www.spectroscopyonline.com 4. https://www.khanacademy.org/science/organic- chemistry/spectroscopy. 5. http://chem.sci.ubu.ac.th/e-learning.