UV VISIBLE SPECTRO Final.pptx
- 1. INSTRUMENTATION OF UV – VISIBLE SPECTROSCOPY
- 2. Absorption spectrophotometry in the ultraviolet and visible region is considered to be one of the oldest physical method for quantitative analysis and structural elucidation. Absorption spectroscopy is the spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. Wavelength • UV- 200-400nm • VISIBLE- 400-800nm 2
- 3. Principle of Uv- Visible Spectroscopy The Principle of UV-Visible Spectroscopy is based on the absorption of ultraviolet light or visible light by chemical compounds, which results in the production of distinct spectra. Spectroscopy is based on the interaction between light and matter. When the matter absorbs the light, it undergoes excitation and de-excitation, resulting in the production of a spectrum. When matter absorbs ultraviolet radiation, the electrons present in it undergo excitation. This causes them to jump from a ground state (an energy state with a relatively small amount of energy associated with it) to an excited state (an energy state with a relatively large amount of energy associated with it). It is important to note that the difference in the energies of the ground state and the excited state of the electron is always equal to the amount of ultraviolet radiation or visible radiation absorbed by it.
- 4. Theory of Uv-visible spectroscopy Ultraviolet and visible radiation interacts with matter which causes electronic transitions (promotion of electrons from the ground state to a high energy state). The ultraviolet region falls in the range between 190-380 nm, the visible region fall between 380-750 nm. The following electronic transitions are possible: π- π* (pi to pi* transition) n - π* (n to pi * transition) σ - σ * (sigma to sigma * transition) n - σ * (n to sigma * transition) and are shown in the below hypothetical energy diagram
- 5. Sigma to sigma * transition (σ → σ∗) A transition of an electron from bonding sigma orbital to higher energy antibonding sigma orbital is designated σ → σ∗ . In alkanes, there are only sigma bonds are available. Therefore, alkenes are showing this type of transition. In general, sigma bonds are very strong. Therefore, high energy is required for σ → σ∗ transition. n to sigma * transition (n → σ∗) n to sigma * transition (n → σ∗) involves saturated compounds with one hetero atom like oxygen,nitrogen, fluorine, chlorine, etc. Normally, saturated halides, alcohols, ethers, aldehyde, ketones, and amines participate in this type of transition. These transitions require comparatively less energy than the σ → σ∗ transition. In saturated alkyl halides, the energy required for n to sigma * transition (n → σ∗) decreases with the increase in the size of the halogen atom or decrease in electronegativity of the atom. Due to the greater electronegativity of chlorine than iodine, the n electron on the chlorine atom is comparatively difficult to excite. The n electrons on the iodine atom are loosely bound.
- 6. pi to pi star transition (π → π∗) pi to pi *transition (π → π∗) in uv vis spectroscopy is available in compounds with unsaturated centers like unsaturated hydrocarbons and carbonyl compounds. It requires lesser energy than n to sigma * transition (n → σ∗). In simple alkenes several transitions are available but the n → π∗ transition required the lowest energy. n to pi * transition (n → π∗) In n to pi * transition (n → π∗), an electron in unshared pair on a hetero atom is excited to π∗ antibonding orbital. It involves the least amount of energy than all types of transition in ultraviolet visible spectroscopy. Therefore, the n → π∗ transition gives the absorption with a longer wavelength. In saturated ketones, n → π∗ transitions around 280 nm are the lowest energy transition. n → π∗ is forbidden by symmetry consideration. Thus the intensity of the band due to this transition is low, although the wavelength is long.
- 8. Lambert’s Law- The amount of light absorbed is proportional to the thickness (length) of the absorbing material (Cuvette) Beer’s law was stated by August Beer which states that concentration and absorbance are directly proportional to each other. The Beer-Lambert law is expressed as: A = εLc where, A is the amount of light absorbed for a particular wavelength by the sample ε is the molar extinction coefficient. The term molar extinction coefficient (ε) is a measure of how strongly a chemical species or substance absorbs light at a particular ... L is the distance covered by the light through the solution c is the concentration of the absorbing species Following are the limitations of Beer-Lambert law: A diluted solution is used There shouldn’t be a scattering of the light beam Monochromatic electromagnetic radiation should be used
- 9. Deviations from Beer Lambert Law Real Deviations – These are fundamental deviations due to the limitations of the law itself. Chemical Deviations– These are deviations observed due to specific chemical species of the sample which is being analyzed. Instrument Deviations – These are deviations which occur due to how the absorbance measurements are made.
- 10. Real Deviation- Beer law and Lambert law is capable of describing absorption behavior of solutions containing relatively low amounts of solutes dissolved in it (<10mM). When the concentration of the analyte in the solution is high (>10mM), the analyte begins to behave differently due to interactions with the solvent and other solute molecules and at times even due to hydrogen bonding interactions. Chemical Deviation- Chemical deviations occur due to chemical phenomenon involving the analyte molecules due to association, dissociation and interaction with the solvent to produce a product with different absorption characteristics. For example, phenol red undergoes a resonance transformation when moving from the acidic form (yellow) to the basic form (red). Due to this resonance, the electron distribution of the bonds of molecule changes with the pH of the solvent in which it is dissolved. Since UV-visible spectroscopy is an electron-related phenomenon, the absorption spectrum of the sample changes with the change in pH of the solvent. Instrumental Deviation- A] Due to Polychromatic Radiation (Also the reason why absorbance measurements are taken at the wavelength of maximum absorbance λmax) Beer-Lambert law is strictly followed when a monochromatic source of radiation exists. In practice, however, it is common to use a polychromatic source of radiation with continuous distribution of wavelengths along with a filter or a grating unit (monochromators) to create a monochromatic beam from this source.
- 11. B] Due to Presence of Stray Radiation Stray radiation or scattered radiation is defined as radiation from the instrument that is outside the nominal wavelength band selected. Usually the wavelength of the stray radiation is very different from the wavelength band selected. It is known that radiation exiting from a monochromator is often contaminated with minute quantities of scattered or stray radiation. Usually, this radiation is due to reflection and scattering by the surfaces of lenses, mirrors, gratings, filters and windows. If the analyte absorbs at the wavelength of the stray radiation, a deviation from Beer-Lambert law is observed similar to the deviation due to polychromatic radiation. C] Due to Mismatched Cells or Cuvettes If the cells holding the analyte and the blank solutions are having different path-lengths, or unequal optical characteristics, it is obvious that there would be a deviation observed in Beer-Lambert law. In such cases when a plot of absorbance versus concentration is made, the curve will have an intercept k and the equation will be defined as: A = εbc + k
- 12. Choice of Solvent The solvent cut off is the wavelength below which the solvent itself absorbs all of the light. So when choosing a solvent be aware of its absorbance cutoff and where the compound under investigation is thought to absorb. If they are close, chose a different solvent. Table .1: UV absorbance cutoffs of various common solvents Solvent UV Absorbance Cutoff (nm) Acetone 329 Benzene 278 Dimethylformamide 267 Ethanol 205 Toluene 285 Water 180
- 13. Solvent Effect- Solvents play an important role in UV spectra. Compound peak could be known by the solvent peak. So a most suitable solvent is one that does not itself get absorbed in the region under investigation. A solvent should be transparent in a particular region. A dilute solution of sample is always prepared for analysis. BATHOCHROMIC SHIFT. The shift of absorption to a longer wavelength due to substitution or solvent effect (a red shift). HYPSOCHROMIC SHIFT. The shift of absorption to a shorter wavelength due to substitution or solvent effect (a blue shift). Hyperchromic: an increase in the molar absorptivity. Hypochromic: an decrease in the molar absorptivity.
- 15. Figure of Absorption Spectroscopy
- 16. PHOTOMETER SPECTOPHOTOMETER COLORIMETER PHOTOMETER: An instrument for measuring the intensity of light or the relative intensity of a pair of lights. Also called an illuminometer. It utilizes filter to isolate a narrow wavelength region. Two types of photometers are used: spectrophotometer and filter photometer. In spectrophotometers a monochromator (with prism or with grating) is used to obtain monochromatic light of one defined wavelength. In filter photometers, optical filters are used to give the monochromatic light. 16
- 17. SPECTOPHOTOMETER: An instrument measures the ratio, or a function of the two, of the radiant power of two EM beams over a large wavelength region. It utilizes dispersing element (Prisms/Gratings) instead of filters, to scan large wavelength region. 17 COLORIMETER: An instrument which is used for measuring absorption in the visible region is generally called colorimeter.
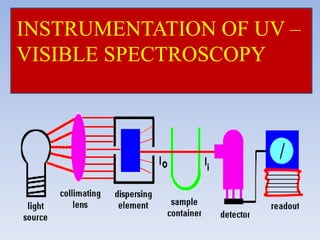
- 18. 18 Suitable amplifier or readout device. Detector system of collecting transmitted radiation Sample holder or container to hold sample. Monochromator and Filter. Source of radiant energy. COMPONENTS OF UV – VISIBLE SPECTROPHOTOMETER
- 19. 19
- 20. 1. SOURCE OF RADIENT ENERGY REQUIREMENTS OF AN IDEAL SOURCE It should be stable and should not allow fluctuations. It should emit light of continuous spectrum of high and uniform intensity over the entire wavelength region in which it’s used. It should provide incident light of sufficient intensity for the transmitted energy to be detected at the end of optic path. It should not show fatigue on continued use. 20
- 21. TUNGSTEN HALOGEN LAMP Its construction is similar to a house hold lamp. The bulb contains a filament of Tungsten fixed in evacuated condition and then filled with inert gas. The filament can be heated up to 3000 k, beyond this Tungsten starts sublimating. It is used when polychromatic light is required. To prevent this along with inert gas some amount of halogen is introduced (usually Iodine). 21
- 22. Sublimated form of tungsten reacts with Iodine to form Tungsten –Iodine complex. Which migrates back to the hot filament where it decomposes and Tungsten get deposited. DEMERIT: It emits the major portion of its radiant energy in near IR region of the spectrum. 22
- 23. I) HYDROGEN DISCHARGE LAMP: (For ultraviolet radiation) In Hydrogen discharge lamp pair of electrodes is enclosed in a glass tube (provided with silica or quartz window for UV radiation to pass trough) filled with hydrogen gas. When current is passed trough these electrodes maintained at high voltage, discharge of electrons occurs which excites hydrogen molecules which in turn cause emission of UV radiations in near UV region. They are stable and robust. 11
- 24. II) Deuterium Lamp (For ultraviolet radiation) If deuterium is used in place of hydrogen the intensity of radiation emitted is 3 to 5 times more The deuterium lamp is more expensive than hydrogen lamp, but it is used when high intensity is required.
- 25. III) XENON DISCHARGE LAMP: (For ultraviolet radiation) 25 It possesses two tungsten electrodes separated by some distance. These are enclosed in a glass tube (for visible) with quartz or fused silica and xenon gas is filled under pressure. An intense arc is formed between electrodes by applying high voltage. This is a good source of continuous plus additional intense radiation. Its intensity is higher than the hydrogen discharge lamp. DEMERIT: The lamp since operates at high voltage becomes very hot during operation and hence needs thermal insulation.
- 26. Mercury Arc Lamp : (For Visible radiation) In mercury arc lamp, mercury vapor is stored under high pressure and excitation of mercury atoms is done by electric discharge. DEMERIT: Not suitable for continuous spectral studies, (because it doesn’t give continuous radiations). 26
- 27. 27 2. FILTERS AND MONOCHROMATORS A source is generally emitting a continuous spectra. Therefore a device is required to select a narrow band from wavelength of continuous spectra. For this selection filter or monochromater or both are used. Following types of monochromatic devices are used. A. Filters B. Monochromater (Prisms and Grating)
- 28. A light filter is a device that allow light of required wavelength to pass but absorb light of other wavelength wholly or partially. Thus a suitable filter can select a desired wavelength band. It means particular filter may be used for a specific analysis. If analysis carried out for several species a large number of filter have to be used and interchanged. Filter are of two type 1. Absorption filters- 2. Interference Filter 28 A. FILTER
- 29. B. MONOCHROMATOR A monochromator successfully isolate band of wavelength usually much more than narrower filter. The essential elements for monochromators are • entrance slit • Dispersing element (Grating or Prism) • Exit slit Material of construction should be selected with care to suit range in which it has to work , e. g. normal glass for visual range, quartz for ultraviolet and alkali halides for IR region
- 30. # Prism Prism is made from glass, Quartz or fused silica. Quartz orfused silica is the choice of material of UV spectrum. When white light is passed through glass prism, dispersion of polychromatic light in rainbow occurs. Now by rotation of the prism different wavelengths of the spectrum can be made to pass through in exit slit on the sample. The effective wavelength depends on the dispersive power of prism material and the optical angle of the prism. 30
- 31. 31
- 32. • There are two types of mounting in an instrument one is called ‘Cornu type’(refractive), which has an optical angle of 60o and its adjusted such that on rotation the emerging light is allowed to fall on exit slit. • The other type is called “Littrow type”(reflective), which has optical angle 30o and its one surface is aluminized with reflected light back to pass through prism and to emerge on the same side of the light source i.e. light doesn’t pass through the prism on other side. 32
- 33. # GRATING Are most effective one in converting a polychromatic light to monochromatic light. It consist of a large number of parallel lines (grooves) ruled on a highly polished surface such as alumina. Generally 15,000 to 30,000 lines per square inch are drawn for UV and Visible region 33
- 34. When light rays are impinged on the grating its grooves act as scattering center for light rays. Thus the light deffracted or spread out over a range of angle and in certain direction, reinforcement or constructive interference may take place. Generally grating are difficult to be prepared. Therefore replica grating are prepared from original grating. This is done by coating the original grating with film of an epoxy resin which is after setting is removed to yield replica. 34
- 35. Grating gives higher and linear dispersions compared to prism monochromator. Can be used over wide wavelength ranges. Gratings can be constructed with materials likes aluminium which is resistant to atmospheric moisture. Provide light of narrow wavelength. No loss of energy due to absorption. 35
- 36. Comparison Prism Grating Made of Glass-: Visible Quartz/fused silica-: UV Alkali halide:- IR Grooved on highly polished surface like alumina. Working Principle Angle of Incident Law of diffraction nλ= d (sini±sinθ) Merits/demerits Prisms give non-liner dispersion hence no overlap of spectral order. It can’t be used over consideration wavelength ranges. Prisms are not sturdy and long lasting. Grating gives liner dispersion hence overlap of spectral order. It can be used over considerable wavelength ranges. Grating are sturdy and long lasting 36
- 37. The cells or cuvettes are used for handling liquid samples. The cell may either be rectangular or cylindrical in nature. For study in UV region; the cells are prepared from quartz or fused silica whereas color corrected fused glass is used for visible region. The surfaces of absorption cells must be kept scrupulously clean. No fingerprints or blotches should be present on cells. Cleaning is carried out washing with distilled water or with dilute alcohol, acetone. 37
- 38. 38
- 39. Device which converts light energy into electrical signals, that are displayed on readout devices. The transmitted radiation falls on the detector which determines the intensity of radiation absorbed by sample The following types of detectors are employed in instrumentation of absorption spectrophotometer 1. Barrier layer cell/Photovoltaic cell 2. Phototubes/ Photo emissive tube 3. Photomultiplier tube 39
- 40. Requirements of an ideal detector:- It should give quantitative response. It should have high sensitivity and low noise level. It should have a short response time. It should provide signal or response quantitative to wide spectrum of radiation received. 40
- 41. The detector has a thin film metallic layer coated with silver or gold and acts as an electrode. It also has a metal base plate which acts as another electrode. These two layers are separated by a semiconductor layer of selenium. 41
- 42. When light radiation falls on selenium layer, electrons become mobile and are taken up by transparent metal layer. This creates a potential difference between two electrodes & causes the flow of current. When it is connected to galvanometer, a flow of current observed which is proportional to the intensity and wavelength of light falling on it. 42
- 43. 43
- 44. 44
- 45. Consists of a evacuated glass tube with a photocathode and a collector anode. The surface of photocathode is coated with a layer of elements like cesium, silver oxide or mixture of them. When radiant energy falls on photosensitive cathode, electrons are emitted which are attracted to anode causing current to flow. More sensitive compared to barrier layer cell and therefore widely used. 45
- 46. The principle employed in this detector is that, multiplication of photoelectrons by secondary emission of electrons. 46 In a vacuum tube, a primary photo-cathode is fixed which receives radiation from the sample. Some eight to ten dynodes are fixed each with increasing potential of 75-100V higher than preceding one. Near the last dynode is fixed an anode or electron collector electrode. Photo-multiplier is extremely sensitive to light and is best suited where weaker or low radiation is received
- 47. 47
- 48. Depending upon the monochromators (filters or dispersing device) used to isolate and transmit a narrow beam of radiant energy from the incident light determines whether the instrument is classified as Photometer or a Spectrophotometer. Spectrophotometers used here detects the percentage transmittance of light radiation, when light of certain intensity & frequency range is passed through the sample. Both can be a single beam or double beam optical system. 48
- 49. • Light from the source is carried through lens and/or through aperture to pass through a suitable filter. • The type of filter to be used is governed by the colour of the solution. • The sample solution to be analysed is placed in cuvettes. 49
- 50. 50
- 51. After passing through the solution, the light strikes the surface of detector (barrier-layer cell or phototube) and produces electrical current. 51 The output of current is measured by the deflection of needle of light-spot galvanometer or micro ammeter. This meter is calibrated in terms of transmittance as well as optical density. The readings of solution of both standard and unknown are recorded in optical density units after adjusting instrument to a reagent blank.
- 52. 52
- 53. 53 Advantage of single beam spectrophotometer Cheap Easy to construct Disadvantage Any fluctuation in the intensity of radiation sources affects the absorbance. Continuous spectrum is not obtained.
- 54. Double beam instrument is the one in which two beams are formed in the space by a U shaped mirror called as beam splitter or beam chopper . 54 Chopper is a device consisting of a circular disc. One third of the disc is opaque and one third is transparent, remaining one third is mirrored. It splits the monochromatic beam of light into two beams of equal intensities.
- 55. 55
- 56. 56
- 57. 57 Advantages of double beam spectrophotometer It facilitates rapid scanning over wide λ region. Fluctuations due to radiation source are minimised. It doesn’t require adjustment of the transmittance at 0% and 100% at each wavelength. It gives ratio of intensities of sample & reference beams simultaneously. Disadvantages:- Construction is complicated. Instrument is expensive.
- 58. SL. NO SINGLE BEAM INSTRUMENT DOUBLBEAM INSTRUMENT 1. Calibration should be Calibration is done done with blank every only in the beginning. time, before measuring the absorbance or transmittance of sample 58
- 59. 2 Radiant energy intensity changes with fluctuation of voltage. It permits a large degree of inherent compensation for fluctuations in the intensity of the radiant energy. 3 It measure the total amount of transmitted light reaching the detector It measures the percentage of light absorbed by the sample. 59
- 60. 4 In single beam it’s not possible to compare blank and sample together. In double beam it’s possible to do direct one step comparison of sample in one path with a standard in the other path. 5 In single beam radiant energy wavelength has to be adjusted every time. In this scanning can be done over a wide wavelength region 6 Working on single beam is tedious and time consuming. Working on double beam is fast and non tedious. 60
- 61. Applications of Uv-visible Spectroscopy 1. Detection of Impurities- Uv- absorption spectroscopy is one of the best methods for determination of impurities in organic compounds. Additional peaks can be observed due to impurities in the sample and it can be compared with that of standard. By also measuring the absorbance at specific wavelength, the impurities can be detected. 2.Structural elucidation of organic compounds Uv-spectroscopy is useful in the structure elucidation of organic compounds, the presence or absence of unsaturation, the presence of heteroatoms. From location of peaks and combination of peaks, it can be concluded that whether the compound is saturated or unsaturated, hetero atoms are present or not.
- 62. 3.Quantitative analysis Uv-spectroscopy can be used for quantitative determination of compounds that absorb uv radiation. 4. Qualitative analysis Uv- absorption spectroscopy can characterize those types of compounds which absorb uv-radiation. Identification is done by comparing the absorption spectrum with the spectra of known compounds. 5. Chemical kinetics Kinetics of reaction can also be studied by using uv- spectroscopy. The uv radiation is passed through the reaction cell and the absorbance changes can be observed. 6.Detection of functional groups This technique is used to detet the presence or absene of functional group.