Keep Manufacturing Facility Trustable
•
0 j'aime•137 vues
Using sound science and risk based approaches is indispensable in prediction and maintaining certainty within emerging regulatory frames.
Signaler
Partager
Signaler
Partager
Télécharger pour lire hors ligne
Recommandé
Recommandé
EnviroCOLA® is a customised cost-saving and compliance support tool for your wastewater treatment and UF-RO plants. EnviroCOLA® collects daily operating parameters from distributed data points, analyses them and provides KPIs which help you ensure compliance and take decisions that save costs. Click on the link below to learn more.EnviroCOLA® – A digital transformation of wastewater treatment and recycling ...

EnviroCOLA® – A digital transformation of wastewater treatment and recycling ...A.T.E. Private Limited
This presentation gives a summary of WHO guidance on Preparation of Laboratory Information File.WHO Guidance on Preparation of Laboratory Information File

WHO Guidance on Preparation of Laboratory Information FileGMP EDUCATION : Not for Profit Organization
This presentation gives a summay of New WHO guidance on Process validation of Non Sterile Pharmaceuticals.Who Guidance on Process Validation for Non Serile Pharmaceuticals

Who Guidance on Process Validation for Non Serile PharmaceuticalsGMP EDUCATION : Not for Profit Organization
Contenu connexe
Tendances
EnviroCOLA® is a customised cost-saving and compliance support tool for your wastewater treatment and UF-RO plants. EnviroCOLA® collects daily operating parameters from distributed data points, analyses them and provides KPIs which help you ensure compliance and take decisions that save costs. Click on the link below to learn more.EnviroCOLA® – A digital transformation of wastewater treatment and recycling ...

EnviroCOLA® – A digital transformation of wastewater treatment and recycling ...A.T.E. Private Limited
This presentation gives a summary of WHO guidance on Preparation of Laboratory Information File.WHO Guidance on Preparation of Laboratory Information File

WHO Guidance on Preparation of Laboratory Information FileGMP EDUCATION : Not for Profit Organization
This presentation gives a summay of New WHO guidance on Process validation of Non Sterile Pharmaceuticals.Who Guidance on Process Validation for Non Serile Pharmaceuticals

Who Guidance on Process Validation for Non Serile PharmaceuticalsGMP EDUCATION : Not for Profit Organization
Tendances (20)
EnviroCOLA® – A digital transformation of wastewater treatment and recycling ...

EnviroCOLA® – A digital transformation of wastewater treatment and recycling ...
How to Prepare for an FDA Inspection and Respond to FDA 483's / Warning Letters

How to Prepare for an FDA Inspection and Respond to FDA 483's / Warning Letters
WHO Guidance on Preparation of Laboratory Information File

WHO Guidance on Preparation of Laboratory Information File
Item 3. Internal quality control - Quality Control Charts

Item 3. Internal quality control - Quality Control Charts
New hire orientation good documentation practice presentation

New hire orientation good documentation practice presentation
Who Guidance on Process Validation for Non Serile Pharmaceuticals

Who Guidance on Process Validation for Non Serile Pharmaceuticals
Similaire à Keep Manufacturing Facility Trustable
FDA Process Validation Guidance (Guidance for Industry: Process Validation- General Principles and Practices, Jan. 2011) outlines process validation activities in three stages - Stage 1: Process Design, Stage 2: Process Qualification and Stage 3: Continued Process Verification. Completion of Stage 2 subsequent to Stage 1 is a major milestone in the Process Validation Lifecycle as it confirms the process design and demonstrates the expected consistent performance of the manufacturing process. Knowledge and information gained from the design stage through the process qualification stage is used to complete this assessment. Stage 2 demonstrates suitability for successful commercial distribution where the data indicates that the process meets the conditions established in the protocol. Continued Process Verification is initiated for the subsequent commercial batches. Stage 3 assures that the process remains in a state of control during commercial manufacture.
This presentation gives a practical approach to implement the stage 3 of the FDA Process Validation Guide.US FDA Process Validation Stage 3: Continued Process Verification

US FDA Process Validation Stage 3: Continued Process VerificationGMP EDUCATION : Not for Profit Organization
Similaire à Keep Manufacturing Facility Trustable (20)
Data integrity Presentation@GCC Regulatory Summit April-2017

Data integrity Presentation@GCC Regulatory Summit April-2017
US FDA Process Validation Stage 3: Continued Process Verification

US FDA Process Validation Stage 3: Continued Process Verification
Plus de Obaid Ali / Roohi B. Obaid
Plus de Obaid Ali / Roohi B. Obaid (20)
A Strategic Talk on Biologicals & Biotechnological Products

A Strategic Talk on Biologicals & Biotechnological Products
Centre for Quality Sciences-Information File-2024.pdf

Centre for Quality Sciences-Information File-2024.pdf
Demonstrate your QMS during Inspection-30 Sep 2023.pdf

Demonstrate your QMS during Inspection-30 Sep 2023.pdf
Track, Trace & Understand Science of Life from Real World Evidence.pdf

Track, Trace & Understand Science of Life from Real World Evidence.pdf
120th Training Session - Unfortunate Wave of DEG Tragedy.pdf

120th Training Session - Unfortunate Wave of DEG Tragedy.pdf
Dernier
Saudi Arabia [ Abortion pills) Jeddah/riaydh/dammam/+966572737505☎️] cytotec tablets uses abortion pills 💊💊
How effective is the abortion pill? 💊💊 +966572737505) "Abortion pills in Jeddah" how to get cytotec tablets in Riyadh " Abortion pills in dammam*💊💊
The abortion pill is very effective. If you’re taking mifepristone and misoprostol, it depends on how far along the pregnancy is, and how many doses of medicine you take:💊💊 +966572737505) how to buy cytotec pills
At 8 weeks pregnant or less, it works about 94-98% of the time. +966572737505[ 💊💊💊
At 8-9 weeks pregnant, it works about 94-96% of the time. +966572737505)
At 9-10 weeks pregnant, it works about 91-93% of the time. +966572737505)💊💊
If you take an extra dose of misoprostol, it works about 99% of the time.
At 10-11 weeks pregnant, it works about 87% of the time. +966572737505)
If you take an extra dose of misoprostol, it works about 98% of the time.
In general, taking both mifepristone and+966572737505 misoprostol works a bit better than taking misoprostol only.
+966572737505
Taking misoprostol alone works to end the+966572737505 pregnancy about 85-95% of the time — depending on how far along the+966572737505 pregnancy is and how you take the medicine.
+966572737505
The abortion pill usually works, but if it doesn’t, you can take more medicine or have an in-clinic abortion.
+966572737505
When can I take the abortion pill?+966572737505
In general, you can have a medication abortion up to 77 days (11 weeks)+966572737505 after the first day of your last period. If it’s been 78 days or more since the first day of your last+966572737505 period, you can have an in-clinic abortion to end your pregnancy.+966572737505
Why do people choose the abortion pill?
Which kind of abortion you choose all depends on your personal+966572737505 preference and situation. With+966572737505 medication+966572737505 abortion, some people like that you don’t need to have a procedure in a doctor’s office. You can have your medication abortion on your own+966572737505 schedule, at home or in another comfortable place that you choose.+966572737505 You get to decide who you want to be with during your abortion, or you can go it alone. Because+966572737505 medication abortion is similar to a miscarriage, many people feel like it’s more “natural” and less invasive. And some+966572737505 people may not have an in-clinic abortion provider close by, so abortion pills are more available to+966572737505 them.
+966572737505
Your doctor, nurse, or health center staff can help you decide which kind of abortion is best for you.
+966572737505
More questions from patients:
Saudi Arabia+966572737505
CYTOTEC Misoprostol Tablets. Misoprostol is a medication that can prevent stomach ulcers if you also take NSAID medications. It reduces the amount of acid in your stomach, which protects your stomach lining. The brand name of this medication is Cytotec®.+966573737505)
Unwanted Kit is a combination of two medicines, whiAbortion pills in Jeddah |• +966572737505 ] GET CYTOTEC![Abortion pills in Jeddah |• +966572737505 ] GET CYTOTEC](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Abortion pills in Jeddah |• +966572737505 ] GET CYTOTEC](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Abortion pills in Jeddah |• +966572737505 ] GET CYTOTECAbortion pills in Riyadh +966572737505 get cytotec
Your voice, smiling, facial expressions, body language, and genuine Hospitality are tightly connected. Always strive to be a Hospitality service professional.
The importance of Smiling is vital in the hospitality industry. People who were or are being raised and educated on digital devices have not had the opportunity to learn and use the necessary and multiple communication skills the way previous generations did. It's a lost art that needs to be presented to the generations that followed the Baby Boomers. These are the generations alive today: Generation X, Generation Y (Millennials), Generation Z, and Generation Alpha.W.H.Bender Quote 62 - Always strive to be a Hospitality Service professional

W.H.Bender Quote 62 - Always strive to be a Hospitality Service professionalWilliam (Bill) H. Bender, FCSI
Dernier (14)
digital Human resource management presentation.pdf

digital Human resource management presentation.pdf
How Software Developers Destroy Business Value.pptx

How Software Developers Destroy Business Value.pptx
internship thesis pakistan aeronautical complex kamra

internship thesis pakistan aeronautical complex kamra
Information Technology Project Management, Revised 7th edition test bank.docx

Information Technology Project Management, Revised 7th edition test bank.docx
Abortion pills in Jeddah |• +966572737505 ] GET CYTOTEC![Abortion pills in Jeddah |• +966572737505 ] GET CYTOTEC](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Abortion pills in Jeddah |• +966572737505 ] GET CYTOTEC](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Abortion pills in Jeddah |• +966572737505 ] GET CYTOTEC
Marketing Management 16th edition by Philip Kotler test bank.docx

Marketing Management 16th edition by Philip Kotler test bank.docx
Gautam Buddh Nagar Call Girls 🥰 8617370543 Service Offer VIP Hot Model

Gautam Buddh Nagar Call Girls 🥰 8617370543 Service Offer VIP Hot Model
Beyond the Codes_Repositioning towards sustainable development

Beyond the Codes_Repositioning towards sustainable development
Siliguri Escorts Service Girl ^ 9332606886, WhatsApp Anytime Siliguri

Siliguri Escorts Service Girl ^ 9332606886, WhatsApp Anytime Siliguri
W.H.Bender Quote 62 - Always strive to be a Hospitality Service professional

W.H.Bender Quote 62 - Always strive to be a Hospitality Service professional
Persuasive and Communication is the art of negotiation.

Persuasive and Communication is the art of negotiation.
Keep Manufacturing Facility Trustable
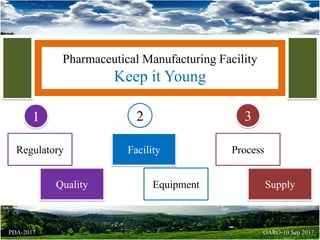
- 1. Pharmaceutical Manufacturing Facility Keep it Young Regulatory Quality Facility Equipment Process Supply 1 2 3 OARO-10 Sep 2017PDA-2017
- 2. Pharmaceutical Manufacturing Facility Keep it Young Regulatory Quality 1 OARO-10 Sep 2017PDA-2017 Review & monitor • Recalls & Lot Acceptance Rates, Trend data & keep an eye on • Increase in Deviations, OOS, CAPAs, complaints, audit observations Trace and track increase in • Change in Process/ equipment/ facility OOS: Out of Specification (Results), CAPA: Corrective & Preventive Action
- 3. Pharmaceutical Manufacturing Facility Keep it Young Facility Equipment 2 OARO-10 Sep 2017PDA-2017 WHEN Finishing attracts Parts are not available Technology is obsolete INCREASE Maintenance & Downtime Contamination issues Environmental Monitoring
- 4. Pharmaceutical Manufacturing Facility Keep it Young Process Supply 3 OARO-10 Sep 2017PDA-2017 Materials • Supplier is lethargic or losing interest Capacity • Increase in market demand Change • ↓ Process yield • ↑ deviations • ↑ Downtime • ↑ Failures • ↑ Variability • ↑ Rework
