Atomic Theory.pptx
- 2. Rutherford’s Gold Foil Experiment ❑ Alpha (α) particles are helium nuclei ❑ Particles were fired at a thin sheet of gold foil ❑ Particle hits on the detecting screen (film) are recorded ❑https://www.youtube.com/watch?v=y_aQWYnbh6 4&list=PL816Qsrt2Os0K7TZ01RZnC9roJvmf40KJ
- 3. Rutherford’s Findings ❑ The nucleus is small ❑ The nucleus is dense ❑ The nucleus is positively charged ❑ Most of the particles passed right through ❑ A few particles were deflected ❑ VERY FEW were greatly deflected “Like howitzer shells bouncing off of tissue paper!” Conclusions:
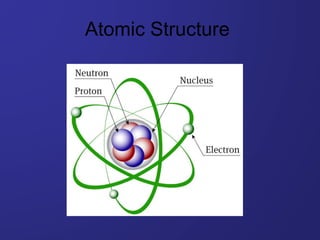
- 4. • Nucleus: Containing protons and neutrons, it is the bulk of the atom and has a positive charge associated with it • Electron cloud: Responsible for the majority of the volume of the atom, it is here that the electrons can be found orbiting the nucleus (extranuclear) The modern atom is composed of two regions:
- 5. Major Subatomic Particles Name Symbol Charge Relative Mass (amu) Actual Mass (kg) Electron e- -1 1/1840 9.11x10-31 Proton p+ +1 1 1.67x10-27 Neutron no 0 1 1.67x10-27 Given in IB data book
- 6. Elemental Classification • Atomic Number (Z) = number of protons (p+) in the nucleus • Determines the type of atom • Li atoms always have 3 protons in the nucleus, Hg always 80 • Mass Number (A) = number of protons + neutrons [Sum of p+ and nº] • Electrons have a negligible contribution to overall mass • In a neutral atom there is the same number of electrons (e-) and protons (atomic number)
- 7. Nuclear Symbols • Every element is given a corresponding symbol which is composed of 1 or 2 letters (first letter upper case, second lower), as well as the mass number and atomic number E A Z elemental symbol mass number atomic number
- 8. Examples • Find the • number of protons • number of neutrons • number of electrons • atomic number • mass number W 184 74 F 19 9 Br 80 35
- 9. Ions • Cation is a positively charged particle. Electrons have been removed from the element to form the + charge. ex: Na loses 1 e- 🡪 Na+ • Anion is a negatively charged particle. Electrons have been added to the atom to form the – charge. ex: Oxygen gains 2 e- 🡪 O2-
- 10. Isotopes • Atoms of the same element that have the same number of protons, but different numbers of neutrons. • The atoms of the same element that differ in the number of neutrons are called isotopes of that element • When naming, write the mass number after the name of the element H 1 1 Hydrogen-1 H 2 1 Hydrogen-2 H 3 1 Hydrogen-3
- 11. How heavy is an atom of oxygen? • There are different kinds of oxygen atoms (different isotopes) • 16O, 17O, 18O • We are more concerned with average atomic masses, rather than exact ones • Based on abundance of each isotope found in nature • We can’t use grams as the unit of measure because the numbers would be too small • Instead we use Atomic Mass Units (amu) • Standard amu is 1/12 the mass of a carbon-12 atom • Each isotope has its own atomic mass
- 12. Calculating Averages Average = (%) x (mass1) + (%) x (mass2) + …. 100 Problem: Silver has two naturally occurring isotopes, 107Ag with a mass of 106.90509 amu and abundance of 51.84 %,and 109Ag with a mass of 108.90476 amu and abundance of 48.16 % What is the average atomic mass? Average = (0.5184)(106.90509) + (0.4816)(108.90476) = 107.87 amu
- 13. • If not told otherwise, the mass of the isotope is the mass number in amu • The average atomic masses are not whole numbers because they are an average mass value • Remember, the atomic masses are the decimal numbers on the periodic table Average Atomic Masses
- 14. Properties of Isotopes • Chemical properties are primarily determined by the number of electrons • All isotopes has the same number of electrons, so they have nearly identical chemical properties even though they have different masses. • Physical properties often depend on the mass of the particle, so among isotopes they will have slightly different physical properties such as density, rate of diffusion, boiling point…
- 15. • Calculate the atomic mass of copper if copper has two isotopes • 69.1% has a mass of 62.93 amu • The rest (30.9%) has a mass of 64.93 amu • Magnesium has three isotopes • 78.99% magnesium 24 with a mass of 23.9850 amu • 10.00% magnesium 25 with a mass of 24.9858 amu • The rest magnesium 26 with a mass of 25.9826 amu • What is the atomic mass of magnesium? More Practice Calculating Averages
- 16. More examples • A sample of boron consists of 10B (mass 10.0 amu) and 11B (mass 11.0 amu). If the average atomic mass of B is 10.8 amu, what is the % abundance of each boron isotope?
- 17. 17 Assign X and Y values: X = % 10B Y = % 11B Determine Y in terms of X X + Y = 100 Y = 100 - X Solve for X: X (10.0) + (100 - X )(11.0) = 10.8 100 100 Multiply through by 100 10.0 X + 1100 - 11.0X = 1080
- 18. 18 Collect X terms 10.0 X - 11.0 X = 1080 - 1100 - 1.0 X = -20 X = -20 = 20 % 10B - 1.0 Y = 100 - X % 11B = 100 - 20% = 80% 11B
- 19. More Examples Copper has two isotopes 63Cu (62.9 amu) and 65Cu (64.9 amu). What is the % abundance of each isotope? (Hint: Check periodic table for atomic mass) 19
- 20. Mass Spectrometer You are not responsible for knowing the steps, but have a general idea of what a mass spectrometer is and what data it outputs!