Rea 2.ppt
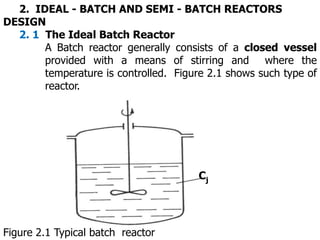
- 1. 2. IDEAL - BATCH AND SEMI - BATCH REACTORS DESIGN 2. 1 The Ideal Batch Reactor A Batch reactor generally consists of a closed vessel provided with a means of stirring and where the temperature is controlled. Figure 2.1 shows such type of reactor. Cj Figure 2.1 Typical batch reactor
- 2. In batch reactor, no material is supplied to or withdrawn from reactor during the reaction the reaction proceeds quickly at first, because of high concentration of all reactants and the composition is only a function of time. due to the well mixing of the composition throughout the vessel.The total mass of the reaction i.e., concentration and temperature are identical over the reactor volume The volume of the batch reactor is the space occupied by the reacting mixture and it may vary during the course of reaction. In gas phase, the volume may be varied as required to maintain the pressure constant.
- 3. t1 t2 t CA,end CA CA,0 XA t Figure 2.2 Variation of concentration with time in a batch reactor Figure 2.3 Variation of conversion with time in a batch Reactor
- 4. CA,0 t=0 t=t1 t=t2 t=tn CA,n Over Reactor Volume Figure 2.4 The concentration inside the batch reactor
- 5. Pressure Gauge Movable Piston I- Constant Volume II- Variable Volume Figure 2.5 Batch reactor for gas phase reaction. The general problem in designing a batch reactor is to calculate the volume of the reactor for a certain average rate of production. The volume can be obtained from the required degree of conversion and the corresponding time. The reaction time of a batch reactor can be determined by using material balance
- 6. 2.1. 1 Design of batch reactors It is important to note that we are dealing a process design not a mechanical design. For process designing a reactor, we need a material balance. Noting that in batch reactor, no material enters or leaves the reaction mixture during the course of reaction, The material balance of equation (1.9) for component A becomes, f V dt A dn dV A r 0 (2.1) We know from the condition that the reaction mixture is perfectly mixed and so no variation in the rate of reaction throughout the reactor volume takes place. Due to this, we can take rA out of the integral and we write the equation (2.1) in the form
- 7. dt A dn f V A r (2.2) and for the disappearance of reactant A, dt A dn f V A r (2.2.1) By integration over the period of the reaction to a final mole, we obtain the basic design equation for a batch reactor. ) ( 0 , 1 A r A dn A n A n f V tr (2.3) In a batch reactor, we are interested in determining how long we should keep the reactants in the reactor to achieve a certain conversion A X A r f V A dX A n r t 0 ) ( 0 , (2.4)
- 8. Equations (2.3) and (2.4) are the general design equations showing, the reaction time required for either constant or variable volume in isothermal or non-isothermal operation. Thus, the volume of reacting fluid and the reaction rate remain under the integral sign, since they both change as reaction proceeds Time involvement of the operation in batch reactor. In completing a batch process, four types of periods are required. These are: •Preparation time tch, during which a reaction is made ready and fresh reactants are charged. •Reaction time, tr, during which the reaction proceeds to the desired conversion. •Recovery time, td, during which the product is discharged and return to the normal operating condition. •Cleaning time, tcl , during which the reactor is cleaned.
- 9. The total time (cycle time), tc, is the time where one operating cycle of a batch reactor is described. cl t d t r t ch t c t (2.5) The capacity of the batch reactor, which is the quantity of the product obtained per unit time is reduced due to this auxiliary time, and it is the major limitation of a batch reactor especially when it is used for a bigger product operation. To carry out the integration of the design equation (2.4.1), we need to know how the reaction rate (-rA) varies with the conversion of the reacting species Generally given (-rA)as a function of conversion (concentration), one can find the time of reaction and eventually also the size of a reactor. We do this by constructing the so called a Levenspiel plot
- 10. 1 (-rA)V Equation (2.4) Area = tr nA,o XA,f XA 1 (-rA) Equation (2.4.1) Area = tr CA,o XA,f XA Figure 2.6 Levenspiel Plots to find the reaction time The integral also can be evaluated using numerical methods such as, Trapezoidal rule, Simpson's rule, Five-point quadratic formula. As for an illustration, the so called Simpson's one-third rule to evaluate the integral f A X A r A X A r A X A r X f A X A r A dX , 1 1 , 4 ) 0 , ( 1 3 , 0
- 11. Relevant in reactor design is a mathematical description that can predict reactor outlet concentration and temperature from inlet concentration, rate of reaction and reactor dimensions. These equations by nature are complicated unless systematically organized and solved. One way of solving such equations is by using algorithm. Algorithm is a rule or calculation procedure for solving a mathematical problem that frequently involves extensive numerical operations. For an example, if we want to design isothermal batch reactor, the algorithm steps will be as under:
- 12. Step1 : We begin by applying our general mole balance equation to arrive at the design equation of the batch reactor. Step2 : We determine the rate equation as the function of concentrations. Step3 : We change the concentration in terms of conversion using stoichiometry relationship. Step4 : Combine the steps 2 and 3 to obtain A X f A r Step5: Evaluate the integral using graphical, numerical, analytical, software package etc. Summarizing the algorithm for batch reactor design gives, Mole Balance and Design Equation. Rate Law. Stoichiometry Combination Evaluation
- 13. 2.1.2 Heat Effect on the batch reactors Generally, all chemical reactions are in principle accompanied by the evolution(exothermic) or absorption (endothermic) of heat. Changes of temperature during reaction are common in reactors This happens deliberately (by heat exchange with the surroundings) or by the nature of the reaction. The temperature change due to the nature of reaction versus conversion is shown in the Figure 2.10 ∆HR = 0 T Conversion, XA Exothermic Reaction Endothermic Reaction Exothermic Figure 2.10 Temperature versus conversion in adiabatic reactors
- 14. Endothermic Exothermic Isothermal Initial reaction Temperature t Figure 2.11 Effect of temperature versus time in a batch reactor In designing a non-isothermal reactor basic design equations i.e., rate laws and stochiometry relationship can be used from the mole balance design equation. The only thing we have to consider additionally is the change of temperature during the reaction
- 15. Let us consider the following highly exothermic reaction carried out adiabatically in a batch reactor B A Our object is to calculate the reaction time for 60% conversion. • Mole balance design equation (2.4.1) gives, f A X A r A dX O A C r t , 0 ) ( , • Rate Law, for first order reaction is A kC A r • Stoichiometry Liquid phase 0 V f V A X A C A C 1 0 , • Combining and canceling the entering concentration o A C , yields f A X A X k A dX r t , 0 ) 1 ( Recalling the Arrhenius equation T T R E k k 1 0 1 exp 0
- 16. Since k is a function of temperature and in adiabatic reaction, temperature is changing during the course of reaction i.e., k is also varying. This forces us not to take k outside the integral, hence the overall equation becomes, f A X A X T o T R E o K A dX r t . 0 1 1 1 exp To solve this equation, we need a relation of XA and T. This type of problems forces us to use both the material and energy balances. To calculate the heat effect in batch reactor, we use the energy balance equation as it is ) ( ) ( T S T KA rV R H dt dT P C T m
- 17. (A) Isothermal Batch Reactor Generally due to the nature of the reaction, the heat is evolved or absorbed during the reaction, thus it is hard to imagine a chemical reaction that has a reaction heat of zero. Normally to bring in isothermal condition, the reaction is brought immediately to that of the surrounding by cooling (if the reaction is exothermic) or heating (if the reaction is endothermic) i.e., the temperature of the reacting mixture is maintained constant through out the course of reaction. This happens with the assistance of the heat transfer medium under perfectly stirring condition. In isothermal operation, there is no change of reaction temperature, i.e., constant temperature (dT = O). If the reactor is to be operated isothermally, the rate of reaction can be expressed as a function of concentration only, not as a function of temperature, then
- 18. ) ( ) ( T S T KA rV R H The material balance can be rearranged dt A dX A n R V A r 0 , Combining the equations gives S T T KA dt A dx o A n R H , The energy balance equation Looking to the above equation, the rate of heat transfer value changes with the rate of reaction. The rate of reaction is changing with temperature. The change of temperature is controlled by the heat transfer equipment, so as to maintain the rate of reaction constant.
- 19. The rate of heat transfer T S T KA Q s T - can be controlled by changing T - by changing over-all heat transfer co-efficient K, which is largely dependant on the agitator speed and the viscosity of the liquid Temperature Controller Steam
- 20. (B) Non – isothermal Batch Reactor This is sometimes called thermal reactors. Two cases During reaction heat absorbed or released, there are deliberate acts of heat introduced or removed from the reactor, which accounts for heat losses to the surrounding. Thus the design of non-isothermal reactors requires simultaneous solutions of the material and energy balance equations There are a lot of questions to be answered to design such type of reactors, such as type of materials of construction, heat transfer equipment etc. The temperature is not constant and varies during the course of reaction, then the rate of reaction will be a function of temperature as well as conversion
- 21. The heat balance for non isothemal becomes, ) ( ) ( T S T KA rV R H dt dT P C T m Taking as an example of a first-order elementary reaction, the rate of reaction becomes, A X A kC A r 1 0 , and the rate constant is defined using Arrhenius Law, RT E A k . exp And rate as a function of both conversion and temperature becomes A X A C RT E A A r 1 0 , exp Substituting the rate equation to the energy balance for non- isothermal operation gives, T S T KA R V R H A X A C RT E A dt dT P C T m 1 0 , exp
- 22. The Equation shows three variables, time of reaction, conversion and temperature. The most important parameter is the reaction time or eventually the size of a reactor for a particular conversion The time of a reaction required for a particular conversion involves simultaneous solutions of the material f A X A r A dx A C r t . 0 0 , and energy balances Simultaneous solution of material and energy balance in analytical form is difficult and numerical methods or analogy simulation must be used. Analytical solution is possible by using the so called trial and error method
- 23. C. Adiabatic Operation Adiabatic reactors operate without any heat addition or removal Based on these facts adiabatic reactors are inexpensive and are used almost in all the industry. Adiabatic reactors can be used when: •The heat of reaction is low where desired conversion and yield can be attained without excessive temperature change. •For high exothermic reaction, inerts or excess of one reactant can be added so that the desired conversion and yield are not adversely affected by excessive temperature change. The energy balance for adiabatic batch reactor without heat transfer equation becomes ) (rV R H dt dT P C T m dt A dX R H A n dt dT P C T m 0 ,
- 24. A dX R H A n dT P C T m 0 , Equation Shows: •Time is not involved •Eliminates the need for simultaneous stepwise solution of the mass and energy balances which was the case for isothermal reaction f T T Differentiation of equation for the initial condition of 0 , A X A X and final condition of and T = TO f A X A X , and 0 ., 0 , 0 A X A X A n R H T T P C T m with the initial condition, 0 0 , A X the equation can be rearranged P C T m A X A n R H T 0 , 0 T - Let P C T m A n R H ad T 0 ,
- 25. ad T is the adiabatic temperature rise of the reaction mixture. This adiabatic temperature rise indicates about how much the temperature of the reaction mixture would rise if the reaction is to proceed adiabatically to completion. Where The relationship between the temperature and the conversion in an adiabatic reactor gives. A X ad T T T 0 A X ad T T T 0 or 0 1 T T ad T A X or For an assumption of , , R H P C are independent of temperature, conversion as a linear function of temperature is shown in Figure ( 2.13 ). The straight line described by equation (2.33.2) intersects the temperature axis at point T = T0 and the slope of such curve becomes.
- 26. 0 , A n R H P C T m b The sign of the slope depends on that of enthalpy change: (-) exothermic,(+) endothermic 1 Material balance Material balance 1 XA XA To T To T Energy balance Energy balance (a) Endothermic b) Exothermic Figure 2.13 Variation of conversion versus temperature
- 27. The adiabatic temperature rise is one of the most important characteristics of the mixture. It's value depends on the initial composition (initial condition) The higher value of adiabatic temperature i.e., the gradient rise is an indication of a stronger thermal effect of the reaction mixture. These thermal effects can be subdued through the dilution of the mixture or by addition of excessive amounts of some of the reactants or inerts. This effect is shown in the Figure (2.14)
- 28. ∆HR > 0 ∆HR < 0 XA To T Isothermal Increase inerts Decrease inerts Decrease inerts Increase inerts Figure 2.14 The effects of addition of inerts to the reaction mixture and the variation of conversion versus temperature in an adiabatic operation.
- 29. 2.1.3 Available Technologies A batch reactor is usually implemented in the industry for small scale operation, mostly for testing new products that have to be fully developed and for the processes that are difficult to convert to continuous operations. The main advantage of batch reactor are high conversion with a long time and The equipment required to conduct a batch operation is relatively simple and encompasses. The disadvantage counts to high labor cost per unit production and the difficulty in obtaining large scale production (A) Reactor It is often a vertical cylinder with an elliptical bottom. The reactor should be constructed in such a way that it can withstand vacuum and some internal pressure. The material used for its construction is generally suitable for various reactionary processes. Most often used is a glass-lined steel vessel.
- 30. This type of reactor is used especially for high corrosion materials. Table 2.2 gives the main characteristics of a standard reactors that are sold commercially. Figure 2.15 shows the illustrations of a simple batch reactor working in the industry. Table 2.2 Main characteristics of standard reactors available in the market
- 31. Capacity (m3) 8 14 25 32 10 20 25 1 4 10 25 0.7 Diameter (m) 2 2.50 2.80 3.10 2.40 2.80 3.00 1.20 1.80 2.40 3.00 0.9 Total weight (kg) 945 0 13,90 0 21,30 0 24,10 0 11000 1800 0 2180 0 1830 2500 5200 7900 800 Exchange area(m2) (1) 18.6 26.6 40.0 45.6 19.8 33 38.8 4.45 10.9 0 11.15 19.5 0 3.4 DE DE DE DE DE DE DE DE DE SE SE DE Service Pressure(bar ) 1.5 1.5 1.5 1.5 6 6 6 6 6 6 6 1.5 Material) AV AV AV AV AV AV AV AI AI AI AI V DE = double jacket, SE = welded external coil, AV = glass-lined steel, AI = Stainless steel, V=
- 32. (B) Heat Exchanger The heat exchanger can be located inside the reactor (coil, plates) outside it (conventional heat exchanger) or could be manufactured together with the reactor wall (double jacket). Figure 2.16 -2.18 illustrates different type of a reactor with heat exchange mechanism Figure 2. 16 Batch reactor with double jacket
- 33. Figure 2.17 Batch reactor with external heat exchange
- 34. Figure 2.18 Batch reactor with double jacket and internal coil
- 35. (C) Stirrer Stirrer is used to mix the reactor contents uniformly so that the concentration remains the same throughout the reactor. There are a number of stirrers used in the technology, such as marine -, turbine-, vane disc-, paddle-, anchor-, double motion-, etc. stirrers. The selection of stirrer type, its dimensions and arrangement are dependant on the type of fluid in the reactor and the stirring is achieved in the operation. (D) Auxiliaries Water, steam, or a heat transfer medium, electrical energy etc. can be used in the operation
- 36. 2.2 Ideal semi-batch reactor design The semi-batch reactor is a tank type operated on a non- steady flow basis. Semi-batch occurs when some reactants are charged into the reactor at time zero, while other reactants are added during the reaction. Figure 2.17 illustrate typical semi-batch reactor. CA,0 Cj Figure 2.19 Semi-batch reactor
- 37. Semi-batch reactor is inevitable when: •It is essential to regulate the heat effect of high exothermic reaction. This can be achieved through regulating the rate of addition of one of the reactants so that the energy evolution can be controlled. •To minimize unwanted side products by maintaining low concentration of one of the reactants, where it is possible to maximize the selectivity of the product especially in the liquid phase reaction. •It is required to remove one of the products to increase the equilibrium of the reaction to the application of Le Chatelier’s principle 1.Material balance For the semi-batch reactor design all the four terms in the general mass balance of equation (1.9) may have to be considered.
- 38. - V dV j r j F - j,0 F dt j dn The mass balance for the reactant A after rearranging becomes V A r A F - A,0 F dt A VC d This equation can be integrated with numerical procedure. Analytical integral is possible for the case of constants of the feed and exit rates of the feed composition; density and additional criteria is that it must be a first-order. dt A VC d V A r - A C f V, F - A,0 C V,0 F For constant densities along constant flow rate dt A C d V V A r - A C V,0 F - A,0 C V,0 F or dt A C d V,0 F V V,0 F V A r - A C - A,0 C
- 39. Using the definition of space time, we have V,0 F V F dt A C d F F A r - A C - A,0 C For a first-order reaction, the rate law is A kC ) ( A r We can combine the rate law and the above equation becomes dt A dC F F A kC - A C - A,0 C Dividing by and rearranging gives, F F A C F dt A dC 0 , k 1 A C This is the linear differential equation, which can be integrated analytically. By doing so, we obtain variation of concentration with time for a first-order reaction in semi-batch operation k 1 - e I k 1 0 , A C F F F A C
- 40. For addition of a substance to a reactor causes to vary the volume of the reactor with time. The reactor volume at any time can be found, using mass balance equation (1.9). The equation (1.9) can be further simplified for this condition as, A,0 F dt V A C d Utilizing the definition of FA,O , we have A,0 C V,0 F dt V A C d or V,0 F 0 dt V d For a constant density system, 0 we have V,0 F dt dV With initial condition V=VO at t=0, by integration gives volume as function of time for semi-batch operation, t V,0 F 0 V V
- 41. 2. Energy Balance The energy balance for semi- batch operation can be written using all items in energy balance of equation( 1.10.3), namely ) ( ) ( ) ( T S T KA E H F H rV R H dt dT P C T m The equation shows the variation involving both composition and temperature as a function of time. Solving such equation is problematic unless we have reduced the variables. ) ( ) 0 ( ) ( T S T KA R T T P C T F rV R H dt dT P C T m or