Name-Reaction-2 (1).pptx
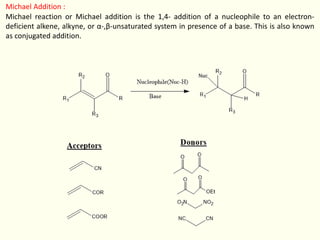
- 1. Michael Addition : Michael reaction or Michael addition is the 1,4- addition of a nucleophile to an electron- deficient alkene, alkyne, or α-,β-unsaturated system in presence of a base. This is also known as conjugated addition.
- 2. Michael Addition : The nucleophiles are called donors.Malonates, nitroalkanes, cyanoacetic esters, acetoacetic esters, and various β-ketoesters are all donors. Electron deficient alkenes or alkynes or α-,β-unsaturated system act as acceptor. Initially, unsaturated carbonyl compounds were utilized, but this was later expanded to include other forms of electron deficient alkenes or alkynes. In general, Michael addition reactions take place in neutral medium at room temperature without the use of a catalyst. The addition of a CH-acidic reagent to the carbon-carbon double bond of an α-,β - unsaturated carbonyl compound is the overall procedure. Michael addition reaction examples Let’s see some examples of this reaction.
- 3. Michael Addition : Michael reaction mechanism Michael reaction mechanism completes in the following steps: Step 1: The base abstracts alpha-acidic hydrogen from the nucleophile to form carbanion which is resonance stabilized.
- 4. Michael Addition : Step 2: The carbanion attacks the electron-deficient compounds. Application of michael addition reaction The Michael addition reaction is a very useful organic reaction and it is used in the synthesis of natural products and drugs in organic chemistry.
- 5. Mannich Reaction : Mannich reaction is a multicomponent condensation between an enolizable carbonyl compound, a non-enolizable carbonyl compound, and an amine to form a β-amino carbonyl compound or β-Amino Ketone or β-amino ester. The enolizable carbonyl compound acts as a donor and the non-enolizable carbonyl compound acts as an acceptor. The amine may be primary or secondary and generally ammonia as well (not tertiary amine). Mostly, formaldehyde is used as a non-enolizable carbonyl compound. Those aldehyde or ketones having alpha-hydrogen are enolizable and those with no alpha- hydrogen are called non-enolizable carbonyl compounds. This reaction can be performed under both acidic and basic condition.
- 6. Mannich Reaction : Mannich reaction mechanism The mechanism of the mannich reaction can be explained in both acidic and basic conditions. A. Mechanism Under the acidic condition Step 1: Nucleophilic addition of amine or ammonia to non-enolizable carbonyl compounds followed by protonation to give an adduct known as iminium ion.
- 7. Mannich Reaction : Step 2: Enolization of the enolizable carbonyl compound.
- 8. Mannich Reaction : Step 3: The iminium ion reacts with enol formed in step 2 to give Mannich base -amino carbonyl compound
- 9. Mannich Reaction : B. Mechanism under the basic condition Step 1: Nucleophilic addition of an amine to a non-enolizable carbonyl compound.
- 10. Mannich Reaction : Step 2: Base abstracts hydrogen from enolizable carbonyl compound to form enolate. Step 3: Reaction between enolate and iminium takes place.
- 11. Mannich Reaction : In organic synthesis, Mannich bases are valuable intermediates. When amine is removed, α,β-unsaturated carbonyl molecule is formed. Another important application of the Mannich base is in alkylation. These are used as alkylating agents. Similarly, the reaction with organolithium and organomagnesium compounds gives β-amino alcohols, which shows pharmacological activities. Mannich reaction plays an important role in Pharmaceutical chemistry. For example, tropinone and alkaloids can be synthesized by using this reaction.
- 12. Diels Alder Reaction : (1928) The Diels alder reaction is an electrocyclic [4+2]-cycloaddition reaction that produces an unsaturated six-membered ring by combining conjugated dienes with dienophiles such as alkenes or alkynes. This reaction is also known as Diels alder cycloaddition reaction. Dienophiles are electron-deficient species, whereas diene is an electron-rich one. As a result, the electron-donating group in the diene favors the reaction, while the electron-withdrawing group on the dinophile speeds up the process The reactivity of dienes is further enhanced by the presence of electron-releasing groups such as alkyl, phenyl, or alkoxy groups.
- 13. Diels Alder Reaction : Dinophiles are electron-poor species such as alkenes and alkynes. One or more electron- withdrawing groups shift electron density away from the Pie-bond in a good dinophile. Some examples of dinophiles are conjugated carbonyl compounds, nitro compounds, nitriles, vinyl ethers, etc. Examples of dinophiles Diels-Alder reaction mechanism is a concerted process in which all bond formation and bond breaking take place at the same time. Mechanism :
- 14. Diels Alder Reaction : It is believed that the mechanism of diels alder reaction involves the radical mechanism in which diradical intermediate is formed. Let’s see such a mechanism. Industrial applications of the diels alder reaction The diels alder reaction is mainly used for synthesizing the six-membered ring. Further, it has been reported that its industrial use in the production of pharmacologically active compounds, agrochemicals, cosmetics, and odors.
- 15. Reformatsky reaction: The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones, with α-halo esters, using a metallic zinc to form β-hydroxy-esters: The organozinc reagent, also called a 'Reformatsky enolate', is prepared by treating an alpha-halo ester with zinc dust. Reformatsky enolates are less reactive than lithium enolates or Grignard reagents and hence nucleophilic addition to the ester group does not occur. The reaction was discovered by Sergey Nikolaevich Reformatsky. Reaction Mechanism Zinc metal is inserted into the carbon-halogen bond of the α-haloester by oxidative addition 1. This compound dimerizes and rearranges to form two zinc enolates 2. The oxygen on an aldehyde or ketone coordinates to the zinc to form the six-member chair like transition state 3. A rearrangement occurs in which zinc switches to the aldehyde or ketone oxygen and a carbon-carbon bond is formed 4. Acid workup 5,6 removes zinc to yield zinc(II) salts and a β- hydroxy-ester 7.
- 16. Reformatsky reaction: Uses of reformatsky reaction The main application of the reformatsky reaction is to prepare β-hydroxy ester from aldehyde or ketones.