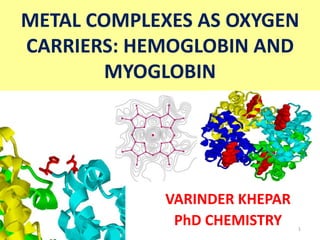
Hemoglobin and myoglobin
- 1. METAL COMPLEXES AS OXYGEN CARRIERS: HEMOGLOBIN AND MYOGLOBIN VARINDER KHEPAR PhD CHEMISTRY 1
- 2. INTRODUCTION • Most of organisms require molecular oxygen for their survival. • For some small animals and plants, where the surface-to- volume ratio is large, the supply of dioxygen can be obtained by simple diffusion across cell membranes i.e. extracted from air or water or photosynthesis. • For other organisms, from scorpions to whales, diffusion does not supply sufficient dioxygen for respiration. • In these organisms specialized molecules for the transport and storage of oxygen are necessary. • These functions are carried out by a number of well-known iron and copper containing species which occur in the blood. These are listed in table. 2
- 3. 3
- 4. METALLOPORPHYRINS • Porphyrins are a group of hetero macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). • Porphyrins are the conjugate acids of ligands that bind metals to form complexes. The metal ion usually has a charge of 2+ or 3+ • They act as tetradentate ligands with 4 N donor sites. • The complexes in which a dipositive metal ion is held in the porphyrin ring system are called metalloporphyrins • Size of centre hole of ring is 0.201nm radius. 4
- 5. 5
- 6. MYOGLOBIN • Was the first protein whose complete tertiary structure was determined by X- tray crystallography. • Has 8 α-helical region and no β-pleated. • Hydrogen binding stabilize the α-helical region. • Consist of a single polypeptide chain includes prosthetic group- one heme group. 6
- 7. • Myoglobin is a relatively small protein of molecular weight of about 17000. • It consists of one polypeptide chain (globin) with one heme group (iron porphyrin complex ) embedded their in. • The peptide chain consists of 150-160 amino acid residues folded about the single heme group. • The heterocyclic ring system of heme is porphyrin derivative containing four pyrrole groups joined by methylene bridges. • The Fe2+ atom present at the centre of the heme is bonded by four porphyrin nitrogen atoms and one nitrogen atom from imidazole side chain of histidine residue which is a part of long protein chain of amino acid residues. • This polypeptide chain plays an important role in biological fixation of O2. 7
- 8. (a) 3-D structure of myoglobin of whale (Physeter catodon) and (b) Structure of the active site of myoglobin 8
- 10. 10
- 11. HEMOGLOBIN • Hemoglobin is a larger protein with a molecular weight of about 64500. • It consists of four sub units each of which contains one heme group associated with protein globin. • There are four heme groups bonded to four protein chains. • One heme group with its protein chain is called sub unit. 11
- 12. • Two sub units form alpha chains of 141 amino acids and two form beta chains of 146 amino acids. • The chains are coiled to form three dimensional structures (also called tertiary structures) are quite similar. • The chain interact with each other through noncovalent interaction – electrostatic interaction, hydrogen bonds, and hydrophobic interaction • Any changes in structure of protein- will cause drastic changes to its property, this condition is called allostery. 12
- 13. ALTERATIONS IN STRUCTURE OF HEMOGLOBIN, LEADING TO DISORDERED FUNCTION OF HEMOGLOBIN REPLACEMENT OF ONE AMINO ACID IN HEMOGLOBIN STRUCTURE LEADS TO DISEASE! Sickle cell anemia – molecular disease of hemoglobin First case described in 1904 – J. Herrick, Chicago physician 13
- 14. 14
- 15. BASIC FUNCTIONS OF HEMOGLOBIN AND MYOGLOBIN • Hemoglobin picks up oxygen in the lungs and delivers it to the rest of the body. • Myoglobin accepts oxygen from the hemoglobin in the muscles and stores it until needed for energetic processes. • Deoxygenated hemoglobin uses some of its amino groups to CO2 back to the lungs. 15
- 16. OXYMYOGLOBIN AND OXYHEMOGLOBIN • As Hb and Mb have five coordinated Fe (II) atom. • It is bonded by four nitrogen atoms from pyrrole rings and fifth from protein chain. • In these complexes, the sixth position is occupied by weakly bonded water. • Mb and Hb in such molecules are usually called as deoxymyoglobin (deoxy-Mb) and deoxyhemoglobin (deoxy- Hb). • When molecular oxygen occupies the sixth position which is trans to histidine chain, then these molecules are called oxymyoglobin (oxy-Mb) and oxyhemoglobin (oxy-Hb). 16
- 17. Illustration of structural changes occurring in myoglobin after oxygen is bound to it 17
- 18. 18
- 19. 19
- 20. 20
- 21. NATURE OF HEME-OXYGEN BINDING • The dioxygen molecule (O2) can bind to iron in heme group in the following three probable ways: • Linear arrangement • Angular or bent arrangement • Sideway symmetrical interaction Fe O O Fe O O Fe O O 21
- 22. OXYGEN TRANSPORT MECHANISM • Hemoglobin and myoglobin play very important role in transporting oxygen from lungs to tissues and CO2 (as HCO- 3 ) from tissues to the lungs. • Oxygen is inhaled into the lungs at very high pressure where it binds Hb in the blood forming HbO2. • The oxygen is then transported to respiring tissues where the partial pressure of O2 is low. 22
- 23. • The O2 then dissociates from Hb and diffuses to the tissues where myoglobin picks it up and stores until it is needed. • Mb has greater affinity for O2 than Hb. This increases the rate of diffusion of O2 from the capillaries to the tissues by increasing its solubility. • The Hb and CO2 (as HCO3 -) are then returned to the lungs where CO2 is exhaled. 23
- 24. COOPERATIVITY • COOPERATIVITY enables the Hb to bind and release oxygen more effectively. • When a substrate binds to one enzymatic subunit, the rest of the subunits are stimulated and become active. • Ligands can either have positive cooperativity, negative cooperativity, or non-cooperativity. • Positive cooperativity is the binding of oxygen to hemoglobin. One oxygen molecule can bind to the ferrous iron of a heme molecule in each of the four chains of a hemoglobin molecule. Deoxy-hemoglobin has a relatively low affinity for oxygen, but when one molecule binds to a single heme, the oxygen affinity increases, allowing the second molecule to bind more easily, and the third and fourth even more easily. The oxygen affinity of 3-oxy-hemoglobin is ~300 times greater than that of deoxy-hemoglobin. This behavior leads the affinity curve of hemoglobin to be sigmoidal, rather than hyperbolic as with the monomeric myoglobin. 24
- 25. • Negative cooperativity means that the opposite will be true; as ligands bind to the protein, the protein's affinity for the ligand will decrease. 25
- 26. Molecular Basis Of Cooperativity • Hemoglobin is a protein with a quaternary structure. It can exist in two quaternary states namely the tense (or T) state and the relaxed (or R) state. • The conformations of the individual polypeptide chains and their relative orientations are different in the T and R states. • Oxygenation rotates the α1β1 dimer in relation to α2β2 dimer about 15°. • When hemoglobin switches from T-state to R state, α1 and α2 globins move closer to each other. • Similarly, β1 and β2 globins move closer in the R-state.These two states are in equilibrium with each other. • Dioxygen can bind to hemoglobin in both the states; however it has a higher affinity for hemoglobin in the R state. 26
- 27. 27 Animation of hemoglobin T-R state transformation
- 28. BOHR EFFECT • In muscles, Hb is much poor O2 binder at lower pressures of O2. • Then Hb passes its O2 on to Mb as required. • The need for O2 is greatest in tissues which have already consumed oxygen and simultaneously produced CO2. • The CO2 lowers the pH and the increased acidity favours release of O2 from oxyhemoglobin to Mb. 2H20 + CO2---------- HCO3 - + H3O+ 28
- 29. • Thus, the oxygen affinity of hemoglobin varies with the pH of the medium This pH sensitivity is called Bohr effect. Thus the variation of oxygen affinity with the pH of the medium is called Bohr effect. • The affinity of Hb for O2 decreases with decreasing pH. • Blood is buffer so that the decrease in pH is very small with accumulation of CO2 in muscles. 29
- 30. • Bohr effect has important physiological effect in transporting oxygen from the lungs to the respiring tissues and in transporting CO2 produced there back to lungs. The CO2 produced diffuses from the muscles tissues to the capillaries and dissolved CO forms bicarbonate only very slowly as : CO2 + 2H20 = HCO3 - + H30+ The enzyme carbonic anhydrase in red blood cells accelerates the reaction so that most of CO2 in blood is carried in the form HCO3 by Hb back to lungs where CO2 is exhaled. 30
- 31. CO Poisoning • Carbon monoxide is a very dangerous gas and can cause fatal poisoning since it binds hemoglobin preferentially over oxygen when both are present in the lungs owing to its higher affinity towards hemoglobin in comparison to oxygen. • Once carbon monoxide sticks to hemoglobin forming a very bright cherry red carboxyhemoglobin, it keeps riding around never giving their seats up to the oxygen. • Eventually, blood loses all of its ability to transport oxygen and there is no way to get oxygen to your brain, heart, or other cells which eventually stops all the biochemical reactions • So, inhalation of even trace amount of carbon monoxide can cause headaches, fatigue, depression and dizziness. However, if exposure is chronic it can lead to more serious complications like heart disease and sometimes death. 31
- 32. 32
- 33. REFERENCES • https://epathshala.nic.in • https://en.wikipedia.org/wiki/Hemoglobin • https://en.wikipedia.org/wiki/Myoglobin • Kalsi P S and Kalsi J P (2016) Bioorganic, bioinorganic and supramolecular chemistry. New age international publishers. 33
- 34. 34