Nyb F09 Unit 3
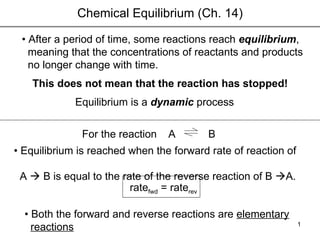
- 1. Chemical Equilibrium (Ch. 14) • After a period of time, some reactions reach equilibrium , meaning that the concentrations of reactants and products no longer change with time. This does not mean that the reaction has stopped! A Equilibrium is a dynamic process B • Equilibrium is reached when the forward rate of reaction of A B is equal to the rate of the reverse reaction of B A. • Both the forward and reverse reactions are elementary reactions For the reaction rate fwd = rate rev
- 2. Rate fwd = k fwd [A] 1 For A B For B A Rate rev = k rev [B] 1 - At equilibrium the rates of forward and reverse reactions are equal so, k fwd [A] = k rev [B] - Rearranging the equation, we get: • Rate laws for elementary reactions are predictable: k fwd k rev = [B] [A] = K The Equilibrium Constant (unitless)
- 4. • Criteria for chemical equilibrium 1. The reaction must be reversible. A reacts to form B, but B can also react to form A 2. The reaction must occur in a closed system. - If the reaction occurred in the open, the carbon dioxide escapes into the environment. The carbon dioxide is no longer present to undergo the reverse reaction. flame CaCO 3 CaO CO 2 Eg. CaCO 3 (s) CaO(s) + CO 2 (g)
- 5. • Because the “concentration” of solids and liquids remains the same throughout a reaction, they do not appear in the equilibrium equation. - In a closed system, equilibrium will establish CaCO 3 (s) CaO(s) + CO 2 (g) K c = [CO 2 ] flame CaCO 3 CaO CO 2 reacts
- 6. The Reaction Quotient: Q • It has the exact same formula as the equilibrium constant and is used to determine reaction direction - If Q = K, then the reaction is at equilibrium - If Q < K, the reaction will proceed forward to produce more products to reach equilibrium - If Q > K, the reaction will proceed in reverse to produce more reactants to reach equilibrium aA + bB cC + dD “ reactants” “ products” (as written) Q = [C] c [D] d [A] a [B] b (reactants) (products)
- 8. In General: 1. When the concentration is changed, the equilibrium will shift to consume some of the added substance or produce some of the removed substance . 2. An increase in pressure (decrease in volume) shifts the reaction to the side with less moles of gas, and an decrease in pressure (increase in volume) shifts the reaction to the side with more moles of gas. 3. For exothermic reactions , an increase in temperature will decrease the K value (shift to the left), and a decrease in temperature will increase the K value (shift to the right). For endothermic reactions , an increase in temperature will increase the K value (shift to the right), and a decrease in temperature will decrease the K value (shift to the left).
- 9. • When it comes to equilibrium in solutions , the volume of the solution itself defines the “closed system” allowing for aqueous reversible reactions to reach equilibrium. Eg. (red/orange) - It is possible to “remove” soluble ions from solution by precipitating them out. Eg. If a hydroxide source is added, it will ppt with Fe 3+ (aq) 500 ml Fe 3+ (aq) SCN - (aq) FeSCN 2+ (aq) +
- 10. Solving Equilbrium Problems: ICE Table • It’s nearly the same as the IRF table for irreversible reactions except that the C stands for “concentration and E stands for “equilibrium”. • ICE tables require the use of variables to fill in unknown information. • Moles can still be entered instead of concentration, but will need to be converted to concentration in the end. 2 HI(g) H 2 (g) + I 2 (g) Problem 1: - At equlibrium, [HI] = 0.078 M. Calculate K. - 0.200 mol of HI gas is added to a 2.00 L flask and the reaction is allowed to proceed at 453 o C
- 11. CO(g) + H 2 O(g) CO 2 (g) + H 2 (g) - 2.00 M of CO and 2.00 M of H 2 O are reacted in a sealed flask at 900 o K. What will be the composition of the reaction mixture at equilibrium? - At this temperature the K is 1.56 • Sometimes the quadratic equation is required to solve equilibrium concentrations - When the equation is rearranged such that: a x 2 + b x + c = 0 Problem 2: - b ± √ b 2 - 4 ac 2 a x = Then
- 12. • When K is a small number (less than ~1.0 x 10 -4 ), a simplifying assumption can be made COCl 2 (g) CO(g) + Cl 2 (g) K = 8.3 x 10 -4 - Calculate the concentration of the reaction mixture at equilibrium when starting with 5.00 mol of COCl 2 in a 10.0 L flask The assumption is considered valid if the percent error is less than 5% Problem 3:
- 13. • The properties of acids and bases have been known for a long time, and many where characteristics back in 1661 by English scientist Robert Boyle. Acid-Base Equilibrium (Ch. 15) Changes the color of litmus dye to blue Changes the color of litmus dye to red Become less basic when combined with acid Become less acidic when combined with base Can have sharp pungent odour Can have sharp pungent odour Have a soapy feel/disposition Are corrosive to metals Have bitter taste Have sour taste Bases Acids
- 14. • Classical, or Arrhenius definition of acids and bases in water: - An acid is a substance that contains H in its formula and dissociates in water to yield H + ions (or H 3 O + , hydronium ion). - A base is a substance that contains OH in its formula and dissociates in water to yield OH - ions . Eg. HCl, HNO 3 , and HCN Eg. NaOH, KOH, and Ba(OH) 2
- 15. • The modern and more accepted view of an acid and base is now called the Br Ø nsted-Lowry acid-base definition: - An acid is a proton donor , any species that donates an H + ion. An acid must contain an H in its formula in order for there to be a possibility of H + donation. - Arrhenius acids are also Bronsted-Lowry acids Lone pair electrons bind the H + HCl(g) + H 2 O(l) H 3 O + (aq) + Cl - (aq) Eg. H Cl O H H Cl - O H H H +
- 16. • A base is a proton acceptor , any species that accepts an H + ion. A base must contain a lone pair of electrons to bind the H + ion, forming a new covalent bond. This is why bases are sometimes also thought of as an electron donor . - This is a very different concept compared to Arrhenius’. For example, Arrhenius would call NaOH a base, but in the B-L definition, it’s the OH - that acts as the base. The compound NaOH is simply the source of the hydroxide ion. N H H H O H H O H + N H H H H - NH 3 (aq) + H 2 O(l) NH 4 + (aq) + OH - (aq) Eg.
- 17. Conjugate acid-base pairs NH 3 (g) + H 2 O(l) NH 4 + (aq) + OH - (aq) base acid NH 3 (g) + H 2 O(l) NH 4 + (aq) + OH - (aq) - However, acid base - The conjugate base has one fewer H and one more minus charge than the acid. - The conjugate acid has one more H and one fewer minus charge than the base. • Conjugate pairs differ by 1 H atom:
- 18. The strength of conjugate acid-base pairs is inversely proportional.
- 19. • pH is a measure of how much H + (H 3 O + ) is in solution pH = - log [ H + ] 1. What is the pH of a solution that is 4.2 x 10 -3 M HCl(aq)? 2. A solution has a pH of 5.5. What is the concentration of H 3 O + in this solution? 3. What is the pOH of a solution of 0.125 M sodium hydroxide? • pOH is a measure of how much OH - is in solution pOH = - log [O H - ]
- 20. The Extent of Dissociation of Weak Acids and Bases HA(aq) + H 2 O(l) H 3 O + (aq) + A - (aq) • For any generic weak acid HA in water, we have - However, since the concentration of water is a constant, it is factored out of the equation to leave a new constant K a , the acid-dissociation constant • Strong acids dissociate 100% into ions (irreversible arrow), but weak acids do not, so their reaction in water is reversible. The bigger the K a , the stronger the acid [H 3 O + ] [A - ] [HA] [H 2 O] K = General equilibrium [H 3 O + ] [A - ] [HA] K a =
- 21. • Similarly, for any weak base B in water, we have the reaction - Again, since the concentration of water is a constant, it is factored out of the equation to leave a new constant K b , the base-dissociation constant The bigger the K b , the stronger the base B(aq) + H 2 O(l) BH + (aq) + OH - (aq) [BH + ] [OH - ] [B] K b =
- 22. Autoionization of Water • Water does not exist purely as the neutral molecule H 2 O. It actually reacts with itself in an Bronsted-Lowry acid- base manner to form ions. To describe this equilibrium, we use the ion-product constant for water, K w K w = [H 3 O + ][OH - ] = 1.0 x 10 -14 (at 25 o C)
- 23. • Some derivations: - Taking the –log of this equation [H 3 O + ][OH - ] = 1.0 x 10 -14 pH + pOH = 14 -log [H 3 O + ] + -log[ OH - ] = -log( 1.0 x 10 -14) K a x K b = K w - For a conjugate pair And so p K a + p K b = 14 (-log K a ) (-log K b ) The smaller the pK a , the stronger the acid
- 24. pH of Soluble Ionic Salts • Review: Salts are solid ionic compounds that consist of ions (cations and anions) in a crystal lattice. Most salt solutions have a pH close to 7.0 Most metal cations do not have any acid or base properties (No H + to donate, and cannot accept an H + either) No H so cannot be an acid (proton donor), but it could be a base (proton acceptor using lone pair electrons). However, it is the conjugate base of the strong acid HCl, so Cl - has negligible base properties. Eg. NaCl(s) Na + (aq) + Cl - (aq) H 2 O
- 25. • Some salts contain the ion of a conjugate base or conjugate acid. Eg. 1.) Sodium acetate: CH 3 COONa (sometimes NaCH 3 COO) 2.) Ammonium chloride: NH 4 Cl - When these types of salts dissolve in water, the pH of the solution will be affected. 1. a) The K a of acetic acid is 1.8 x 10 -5 . What is the K b for its conjugate base acetate? b) 0.15 moles of sodium acetate is dissolved in a total of 1.0 L of water. What is the pH of the solution? 2. The K b for ammonia is 1.8 x 10 -5 . What would the pH be of a solution of 0.23 M ammonium chloride?
- 26. Acid-Base Buffers • An acid-base buffer is a solution that resists changes in pH when a small amount of strong acid or base is added. - a solution can act as a buffer it in contains an acid- base conjugate pair , in similar concentrations • Buffer range: Buffers work best when there are equal concentrations of conjugate pairs. When this occurs, the pH = pK a A buffer is effective at a pH within approximately ± 1 of the pKa • Buffer capacity: The more highly concentrated the buffer solution, the more acid or base it can “consume”. Buffer pH is NOT affected by addition of water
- 27. H 2 O + CO 2 H 2 CO 3 HCO 3 - + H + Carbonic acid Bicarbonate • A Biological Buffer: Controlling pH in blood plasma Controlled through respiration Controlled by the liver Basic substances are consumed by the carbonic acid, and acidic substances are consumed by the bicarbonate.
- 28. • Ways of Making Buffers 1. Adding both conjugates into solution . 2. Generating the conjugate pair through neutralization (more common method) - The ionic species typically comes from a salt. Eg. Acetic acid (solution) with sodium acetate . Ammonia (solution) with ammonium chloride. - When a weak acid is partially neutralized by a strong base, a certain quantity of conjugate base will form. (Weak bases require partial neutralization by strong acids). Eg. CH 3 COOH(aq) + NaOH(aq) CH 3 COO - (aq) + Na + (aq) + H 2 O
- 29. A buffer is made by dissolving 7.38 grams of solid sodium acetate to a total of 1.0 L solution with 0.100 mol of acetic acid. What is the pH of the buffer solution? Formula weight of sodium acetate = 82.03 K a of acetic acid is 1.8 x 10 -5 1. a) b) Calculate the pH if 500.0 ml of water is added to the buffer. c) Calculate the pH if 0.400 g of solid sodium hydroxide is added to the buffer in a) Formula weight of sodium hydroxide = 40.00 d) Calculate the pH if 0.400 g of solid sodium hydroxide is added to 1.0 L of pure water.
- 30. 2. You want to make 1.00 L of a 0.100 M solution buffered at pH 9.0 using an ammonia/ammonium buffer system. K b of ammonia = 1.74 x 10 -5 a) How many moles of each conjugate species would you need? b) The source of ammonia is a 1.00 M solution, and the source of ammonium is from solid ammonium nitrate. (FW = 80.04). How much would you add of each to achieve your buffer? 3. You want to make 1.00 L of a 0.100 M solution buffered at pH 9.0 using using an ammonia/ammonium buffer system. You have only solid ammonium nitrate and 6.00 M NaOH solution. How much of each must be used?
- 31. Titration Curves • During an acid-base titration, the pH of the solution changes as acid or base is continuously added. • The plot of the pH versus the quantity of acid or base added, generates a titration curve . Important points on the titration curve: 1.) Equivalence point: The mid point of large inflections in the curve. (“S” portion of the curve) 2.) Half equivalence point for weak acids and bases : This is when pH = pKa
- 32. Automated pH Titration Data Acquisition Example: Strong acid HCl titrated with NaOH
- 34. HAc = acetic acid Ac - = acetate ion
- 36. Polyprotic Weak Acids • These acids have multiple ionizable protons (acidic hydrogens) Eg. H 3 PO 4 – Phosphoric acid has 3 acidic hydrogens H 2 CO 3 – Carbonic acid has 2 acidic hydrogens H 2 SO 4 – Sulfuric acid has 2 acidic hydrogens • Interestingly, for weak polyprotic acids, the protons ionize one at a time . The first proton has the greatest ionizability, followed by the next proton, etc. H 2 SO 3 HSO 3 - SO 3 2- K a1 K a2 Where K a1 >> K a2 Nearly all polyprotic acids are weak acids except for sulfuric H + H +
- 38. Molecular Bases are N Containing Compounds
- 39. Equilibria of Slightly Soluble Ionic Compounds • On solubility table, these are compounds marked as “ss” Eg. CaSO 4 (s) Ca 2+ (aq) + SO 4 2- (aq) • In solution, small quantities of solid are dissolving into ions. Once a certain ion concentration is reached, the ions react to reform the solid in an equilibrium General K [ Ca 2+ ] [ SO 4 2- ] [ CaSO 4 ] = K sp = [ Ca 2+ ] [ SO 4 2- ] Where K sp stands for the solubility product constant
- 40. • Shifting solubility is a property of Le Chatelier’s Principle - This will increase the concentration of “products” in solution and shift the equilibrium to the left. - The result is that the salt is even less soluble in a solution containing a common ion. 2.) pH differences - excess H 3 O + (low pH) can often react with one of the ions of the salt, removing it from solution. - This will reduce the concentration of “products” in solution, shifting equilibrium to the right which increases the solubility of the salt. - Excess OH - (high pH) often acts as a common ion 1.) A common ion in solution.
Notes de l'éditeur
- An non-chemical analogy for an equilibrium situation is with population. Say the island of Montreal has a population of 2.5 million people, and this number hasn’t changed for the last 3 years. Intuitively, you probably realize that the reason the number hasn’t change is not because no one is moving or no one is dying or being born. It is sure that these things are still happening. If so, how does the population remain “unchanging”? The factors that would reduce population (death and moving out of the island) must be balanced by the factors that increase population (birth and moving into the island). Another way to put it is that equilibrium is created from a balance of rates. Rate of loss of population = Rate of gain When it comes to chemical equilibrium, the concept is very similar. The rate of the forward reaction is equal to the rate of the reverse reaction, creating a particular ratio of products to reactants at any given time.
- This slide essentially explains how the K equilibrium constant is established: it’s the ratio of the forward rate constant over the reverse rate constant, OR more simply, the concentration of products over the concentration of reactants.
- Keep in mind that the reactions are reversible, and can actually be written the other way. This makes the K of the reverse reaction (call it Krev) the inverse of Kfor. For example, if the K for a particular reaction is 25.0. The K for the reverse reaction is 1/25.0 = 0.04.
- Note that in the textbook, they refer to the equilibrium constant as Kc, where the subscript c stands for concentration. So for the reaction of calcium carbonate, the equilibrium constant would be: Kc = [CO2] ONLY SUBSTANCES THAT CAN CHANGE IN CONCENTRATION AFFECT EQUILIBRIUM. Concentrations of solids and liquids don’t change since the substance itself defines the volume . Take 1.0 L of liquid water. It weighs 1000 grams, which equals 55.56 moles. This many moles in 1.0 L gives water a concentration of 55.56 M. What happens if we boil away 500.0 ml of water? What will be the concentration now? As you can see, we’ve reduced the moles of water by half. However, we’ve also reduced its volume by half! The liquid water is still 55.56 M, and will always be this concentration regardless of quantity.
- Calculating Q is exactly the same as the definition of the equilibrium constant K. Q is used when the chemical situation may not be at equilibrium. When Q is compared to K, we can see how the reaction will change over time to achieve equilibrium. Example: Problem 17.5 For the reaction N2O4(g) 2 NO2(g), the K = 0.21. You add 0.12 mol of N2O4 and 0.55 mol of NO2 into a 1.0 L flask and seal it. Is the reaction at equilibrium? If not, which way will the reaction proceed to reach equilibrium? Q = 0.552/0.12 = 2.5 This number is much bigger than K. Equilibrium will be established when more N2O4 reactant is produced. Therefore, the reaction will proceed right (to make more reactant) over time to reach equilibrium.
- For the reaction PCl3(g) + Cl2(g) PCl5(g) We can see that the K for this reaction is = [PCl5]/[PCl3][Cl2], and let’s pretend that the K is 1.0 to make the math easier Changes in concentration What would happen to this reaction if all of a sudden we added more PCl3(g) to the reaction system? In general, the equilibrium system reacts to consume some of the added substance or produce some of the removed substance. Since we added reactant the reaction will shift to the right to try to consume it (get rid of the excess). Let’s use some simple numbers to see what this sudden stress on the system does. Say the concentration of all three compounds in the above reaction is 1.0 M. PCl3(g) + Cl2(g) PCl5(g) At equilibrium: 1.0 M 1.0 M 1.0 M making K = 1 Now let’s say that the injection of extra PCl3 has increased its concentration to 2.0 M. What has happened to the ratio of products to reactants? We can use reaction quotient, Q to help us predict the shift in equilibrium. Q=[PCl5][PCl3] [Cl2] Q=1.0 M(2.0 M)(1.0 M) = 0.50 We can see that Q < K. This means the top number is too small. The reaction needs to make more product such that Q can equal K. This is the mathematical explanation for the system shifting to the right to try to consume excess added substance. Changes in concentration can also be achieved by removal of a reactant or product. Let’s say Cl2(g) was able to be removed from the reaction mixture, reducing its concentration. The reaction will shift to the left, to produce more of what’s been removed. The effect of changing pressure (by changing total volume) Of course, pressure changes will have an effect only on gases, since solids and liquids are incompressible. Let’s see what happens to the same reaction as above in a system where we can alter to total pressure. Movable pistonHigh pressure (small volume)Reaction in Equilibrium 1.0 LLow pressure (Large volume) 2.0 L 0.50 L Say we have these quantities of gases in 1.0 L volume PCl3(g) + Cl2(g) PCl5(g) 1.0 mol 1.0 mol 1.0 mol 1.0 M 1.0 M 1.0 M Let’s see what would happen to the system if the piston moved down, reducing the volume to 0.50 L. There is still 1.0 mol of each substance but now in a new volume of 0.50 L, all the concentrations have become 2.0 M.Q=2.0 M(2.0 M)(2.0 M) = 0.50 Q < K so the reaction shifts to the right to form more products, essentially reducing total gas volume and restoring the pressure. Another way to look at it is that there are 2 molar equivalents of gas on the reactant side, whereas there’s only 1 molar equivalent of gas on the product side. An increase in pressure (decrease in volume) shifts the reaction to the side with less moles of gas. Now if the piston is moved up to make the volume 2.0 L, all the concentrations become 0.50 M.Q=0.50 M(0.50 M)(0.50 M) = 2.0 Q > K so the reaction shifts to the left to form more reactants. An decrease in pressure (increase in volume) shifts the reaction to the side with more moles of gas. What would happen in the same piston-vessel above with this reaction: H2(g) + I2(g) 2HI(g) 2 mole gas 2 mol gas In terms of pressure, there is no way that the reaction could shift to relieve the pressure, so nothing happens. In terms of concentrations, you can see that all the concentration changes will occur in the same proportions of product to reactant, so the K value has not changed. Therefore, if both sides of the equation have an equal number of moles, the reaction does not shift. The effect of changes in temperature To know how a change in temperature will affect the equilibrium of a reaction, we have to know the enthalpy of the reaction, ΔH , or the heat involved with the reaction process. If the ΔH is negative, the reaction is exothermic and releases energy into the surrounding environment during the reaction. If the ΔH is positive, the reaction is endothermic and requires energy to occur. The energy the reaction requires needs to be drawn from the surrounding environment for it to occur. PCl3(g) + Cl2(g) PCl5(g) ΔH0rxn = -111 kJ/mole The forward reaction is a favorable one that will release heat. What happens if we increase the temperature of this reaction? Since release of heat is a requirement for the reaction, increasing the temperature hinders the heat loss and will slow down the forward reaction and favor the back reaction . However, if we lowered the temperature, the loss of heat from the reaction will occur more readily, and therefore the forward reaction will occur to a greater extent. The exact opposite happens for an endothermic reaction. The reaction requires heat energy to occur, so increasing the temperature favors the forward reaction, and reducing the temperature reduces the forward reaction. The difference with the temperature effect is that the favorability of the reaction to form the products or reactants has been altered, and in doing so changes the concentrations of the reactants and products at a new equilibrium position, thereby changing the K value . It is the only factor that changes the K value of the reaction. Changes in concentration and changes in pressure do not change the K value. For exothermic reactions, an increase in temperature will decrease the K value (shift to the left), and a decrease in temperature will increase the K value (shift to the right). For endothermic reactions, an increase in temperature will increase the K value (shift to the right), and a decrease in temperature will decrease the K value (shift to the left).
- This example is done using concentration in the IRE table. Concentration (M) 2HI(g) H2(g) + I2(g) Initial I: 0.100 0 0 Reaction R: -2x +x +x Equilibrium E: 0.100-2x x x Since the reactants are initially at 0, it is for certain that the reaction will proceed to the right to achieve equilibrium. This is why the reactant is decreasing by 2x, whereas the products are increasing by x according to the stoichiometry. The E line represents the concentrations of the entire reaction composition at equilibrium. Therefore, we need to solve for x.
- x = 1.11 M and now all concentrations can be calculated. Try the same question except starting with 2.00 M CO(g) and 1.00 M H2O 0.56 x2 – 4.68 x + 3.12 = 0 To solve for x, you have no choice but to use the quadratic formula. x = 7.6 M and/or 0.73 M. However, the 7.6 M doesn’t work since it results in a negative concentration for CO and H2O.
- The qualification of a small K is approximate. It also depends on the quantity of the reactants. The fact that K is small means that when equilibrium is established, there is a very low quantity of products compared to reactants. The forward reaction is not favored. Concentration (M) COCl2(g) CO(g) + Cl2(g) Initial I: 0.500 0 0 Reaction R: -x +x +x Equilibrium E: 0.500-x x x The assumption: since K is small, x will also be very small such that 0.500-x ~ 0.500. We are assuming that x is negligible, kind of like 1 million dollars minus 1 dollar. It’s still essentially 1 million dollars. Now the K equilibrium expression will be simplified and we can avoid using the quadratic formula to solve x. Solving for x gives 0.0204. Now, is 0.0204 really small enough such that 0.500-0.0204 is considered ~0.500? The assumption is valid within 5% error so… Therefore the assumption is valid.
- The classical definition is the most obvious way to think of acids and bases. However, the definition has a flaw when it comes to defining a weak base. For example, what is NH3? An acid or a base? According to the classical definition above, if it is not a neutral compound, NH3 looks as though it could be an acid since it contains H in its formula. But by all observable properties ( slide 2 ), it is clearly a base, yet it does not contain OH in its formula. How can this be?
- Clearly, a new concept had to be developed to define a compound such as NH3 as a base. TWO THINGS TO NOTE: 1.) The terms “proton” and “hydrogen ion, H+” mean the same thing and often times are used interchangeably. Why is the H+ ion called a proton? Hydrogen is a unique element in that it has only one proton in its nucleus and no neutrons. The H atom also has only one electron. The H+ ion is created when the hydrogen atom loses an electron. What does the H+ ion have left? Just a single proton: the H+ ion is essentially nothing more than a single proton! 2.) A lone pair of electrons surrounding an atom are valance electrons that complete the atom’s octet, but are NOT involved in covalent bonding.
- you can see why NH3 is a base: when added to water, it produces OH- in solution.
- Notice that the reaction is reversible. When the reaction goes backwards, we have new chemical species that also have an acid and base, as defined by the Bronsted-Lowry definition. Therefore, NH3 and NH4+ are considered a conjugate pair, as well as H2O and OH-. Exercise: match up conjugate pairs from the following list: Cl-, OH-, CH3COOH, NH4+, H2SO4, H3O+, CH3COO-, NH3, H2O, SO42-, HCl, Na+, HSO4-
- Calculating the extent of dissociation of acids and bases For acids and bases, the amount of H3O+ and OH- they can produce in solution indicate how strong of an acid or base they can be respectively. In chemistry, HCl is often used as your model strong acid. The model weak acid used is often acetic acid, CH3COOH. Because we know that strong acids dissociate 100% into ions, it is easy to determine how much H3O+ is produced given the concentration of the acid. A 0.10 M solution of HCl would react as follows: HCl(aq) + H2O(l) H3O+(aq) + Cl-(aq) 0.10 M 0.10 M 0.10 M According to the equation, we have proof that HCl dissociates 100% into ions The pH of a 0.10 M solution of HCl(aq) would be 1.0 How much H3O+ is produced from a 0.10 M solution of acetic acid? What would be the pH of the solution? Since this is a reversible reaction in solution, the reaction will reach equilibrium, and the extent of the forward reaction to produce products will be dependent on its acid-dissociation constant Ka. For acetic acid, Ka = 1.8 x 10-5 CH3COOH(aq) + H2O(l) H3O+(aq) + CH3COO-(aq) I 0.10 M R -x +x +x E 0.10 M x x By solving for x, we will know the concentration of H3O+ produced from a 0.10 M solution of acetic acid. At first glance, it looks like we will have to solve a quadratic formula after cross multiplying. However, what do you notice about the value for Ka? It is a very small number, meaning that the forward reaction does not proceed to a great extent. Therefore, we can assume that x will be small such that 0.10 – x ~ 0.10 The equation simplifies to: Now solving for x is simple: x2 = 1.8 x 10-6. x = 1.3 x 10-3 M = [H3O+]= [CH3COO-] pH = 2.9 Using the formula above, we get a percent dissociation of only 1.34 %.
- Since self-ionization is an inherent property of water as defined by the Kw expression, it means that there is always going to be H3O+ ion and OH- ions in dilute aqueous solutions . This concept is very important for you to understand. The concentrations of these ions however, will be changed by addition of either acid or base to water. Question: A 0.01 M solution of HCl is made. What is the concentration of OH- in this solution? 0.01 M of HCl will generate 0.01 M of H3O+ (100% ionization). Using the Kw equation, we get: [OH-] = Kw/[H3O+] = 1 x 10-14/0.01 M = 1 x 10-12 M. The OH- concentration is extremely small, but there is still some in solution.
- Proof of the bottom half of the slide: So for any generic acid HA in water we have the reaction: HA(aq) + H2O(l) H3O+(aq) + A-(aq) Ka = [H3O+][A-] [HA] A- is the conjugate base of the acid HA. A- will therefore act as a base in solution. The reaction of the conjugate base A- in water gives: A-(aq) + H2O(l) OH-(aq) + HA(aq) Kb = [OH-][HA] [A-] When we multiply the two equilibrium constants: Ka x Kb = [H3O+][A-] x [OH-][HA] [HA] x [A-] You can see that the same species in both the numerator and denominator cancel out, leaving behind: Ka x Kb = [H3O+][OH-] = Kw Analogous to the invention of the pH scale for convenience, we can also represent the Ka and Kb values in numbers that are easier to work with. These are the pKa and pKb. pKa = -logKa and pKb = -logKb
- 1. a) Kb (of acetate) = Kw/Ka (of acetic acid) Kb = 5.56 x 10-10 b) Dissolving 0.15 mol of sodium acetate will produce 0.15 mol of acetate ion. NaCH3COO(aq) Na+(aq) + CH3COO-(aq) 0.15 mol 0.15 mol 0.15 mol The acetate ion will react with water reversibly and create an equilibrium CH3COO-(aq) + H2O(l) CH3COOH(aq) + OH-(aq) I 0.15 M excess 0 0 R -x +x +x E 0.15 – x x x Since the Kb is super small, x will be extremely small such that 0.15 – x ~ 0.15 Now the equilibrium equation becomes 5.56 x 10-10 = x2/0.15 Solving for x gives 9.13 x 10-6 M, which happens to be the concentration of OH- in the sodium acetate solution. Since Kw = [H3O+][OH-], solving for [H3O+] gives 1.10 x 10-9 M. Therefore pH of the sodium acetate salt solution = 8.96 2.) pH of the ammonium chloride solution = 4.95
- A particular acid-base buffer system is effective at resisting pH changes with a certain pH range, also called the buffer range . If there are equal quantities of acid and conjugate base for a buffer… Ka = [H3O+][A-] If [A-] and [HA] are the same number, you can see that they will cancel out and [HA] Ka = [H3O+] When taking the –log of both sides of the equation, pKa = pH. Say you made an acetic acid/acetate buffer solution. At what pH is the buffer most effective? Acetic acid has a Ka = 1.8 x 10-5, so it’s pKa is 4.7. Therefore, an acetic acid/acetate buffer solution is most capable of resisting pH changes in the range of 4.7 plus or minus 1 pH value. Capacity. Eg a 1.0 M buffer can neutralize more than a 0.10 M buffer.
- The buffer concept is extremely important in biology. Molecules such as enzymes, proteins, DNA, fats, etc. within the cells of living organisms function optimally at near neutral pH. Slight fluctuations in pH could destroy molecular function, leading to death of the cell, and eventual death of the organism. Therefore, it is absolutely crucial for cellular fluids to maintain a particular pH. Shown in the slide are the chemical components that regulate blood pH to keep it very close to 7.4. This is the main buffer system that soaks up lactic acid produced by muscle cells during vigorous (anaerobic) exercise. It is believed that one of the main reasons vigorous activity cannot be sustained indefinitely is because of the lowering of pH by production of excess lactic acid during vigorous exercise. If you take a break, allow the buffer system to restore the pH balance, another burst of vigorous activity can be achieved.
- Method #1 is fairly obvious. Since we need both the acid and conjugate base in the same solution, we simply find chemicals that contains both sources. For example, to make an acetic acid/acetate buffer, we would simply find some acetic acid solution, and a salt that contains acetate ion, such as sodium acetate. Method #2 is equally common to use. Say we have 1.0 mol of acetic acid, but no sodium acetate. The acetate ion can be generated by reacting with NaOH. Acetic acid and sodium hydroxide will neutralize each other to produce a salt and water. However, we don’t want to neutralize all the acetic acid. Optimally, we would neutralize about half the quantity of the acid. CH3COOH(aq) + NaOH(aq) CH3COO-(aq) + Na+(aq) + H2O I 1.0 mol 0.50 mol 0 0 0 R -0.50 mol -0.50 mol +0.50 +0.50 +0.50 F 0.50 mol 0 0.50 mol 0.50 mol 0.50 mol Now with equal quantities of acid and conjugate base in solution, an equilibrium will be achieved to create a buffer.
- Question 1 is a “forward” buffer calculation problem. You’ve made a buffer by doing this and that, now calculate the pH. a) Sodium acetate is a salt that dissociates 100%: NaCH3COO(aq) Na+(aq) + CH3COO-(aq) - Solve for moles of sodium acetate: 7.38 g / 82.03 g/mol = 0.0900 mol This means that you’ve added 0.900 mol of acetate to the solution. - The moles of acetic acid present is 1.0 L x 0.10 M = 0.10 mol The acetic acid and acetate create an equilibrium reaction in solution as shown below. Traditionally, concentration values are entered into the IRE table. I will enter in the “concentrations” of each aqueous species, but as an undivided mol/L term for now. It will make more sense as to why I’m doing this a bit later. The reaction proceeds to the right to form H3O+ in order to reach equilibrium. The new concentrations of each species at equilibrium are shown on the E line. To simplify the math and avoid using a quadratic equation, you may state that since Ka is small, x will be small such that This way, the equilibrium expression simplifies to Now, back to the reason why I didn’t simply divide by the volume to calculate the concentration. I wanted you to see that in buffer calculations, because the moles of all the chemical species are “swimming” around in the same volume, the volume term cancels out of the equation! The pH of the buffer solution is essentially dependent on the MOLAR ratio of conjugate acid and base. Solving for x gives 2.0 x 10-5 M, which is the concentration of H3O+ in the buffer (Note that if you check percent error, the assumption holds). Therefore, the pH of this buffer is 4.7 b) This is simply to show you that buffer pH is not affected by addition of water (unless you make the buffer too dilute. Then the pH will start to change.). If 500 ml of water is added, the moles of acetic acid and acetate are now “swimming” around in a larger volume, 1.5 L. Here’s now the final equation to calculate pH will look: Again, the volume terms cancel out, illustrating once again, it is the molar ratio of conjugate acid and base that determine the pH of solution. So the pH adding water is still 4.7 c) When a base, NaOH is added to the buffer solution, the first thing that occurs is an irreversible neutralization reaction. An IRF table is required. Next, you will have to set up the table with the correct neutralization reaction. Hopefully, it should be obvious that the acid component, CH3COOH will be the species that neutralizes the added base. - moles of NaOH added: 0.400 g / 40.00 g/mol = 0.0100 mol The neutralization reaction: Notice the quantities entered in the I line are the pre-existing numbers calculated earlier in part a). I also wrote in 0 for Na+ and H2O although they really aren’t. It’s because these numbers are inconsequential to the pH of the final solution. The F line shows the new concentrations of the species once the neutralization reaction is over. Now we have 0.090 mol acetic acid and 0.10 mol acetate “swimming” around in a certain volume of solution, and the equilibrium reaction will once again re-establish. I put “some V” since the volume term will cancel out in the final equation after the simplifying assumption is made. Solving for x gives 1.62 x 10-5 M. Therefore the pH is now 4.8. This goes to show the whole purpose of a buffer solution. It resists pH changes when strong acid or base are added. The original pH was 4.7, and after adding some NaOH, the pH became 4.8, which is a fairly small change. d) If we add the same quantity of NaOH to regular, unbuffered water (pH 7.0), the pH change will be drastic. 0.01 mol of NaOH(aq) will generate 0.01 mol of OH- ions. In a volume of 1.5 L, the concentration of OH- will be 00067 M. This gives a pOH of 2.2, or a pH of 11.8. The water has gone from 7.0 to 11.8, which is a very significant change. Any biological system (cells, bacteria, algae, proteins, etc), will quickly die or deteriorate.
- The main reaction occurring in an acid-base titration is a neutralization reaction. Let’s take a titration of HCl(aq) with NaOH(aq) as an example. The pH of the solution at any time can be measured by a device called a pH probe. The data can also be recorded automatically if the probe is interfaced with a computer program such as Labworks. The set up would look something like this:
- The titration curve of a strong acid with a strong base will have this general shape. (The titration of a strong base with an acid would generate the inverse curve). The pH at any point in the curve is dependent on what chemical species are in solution. It is important that you realize what chemical species are present, and which ones have an impact on the pH. Characteristics of this curve: stays at low pH for extended period. The pH rises rapidly near equivalence. The vertical region of the curve long (large “S” shape). pH at equivalence is 7.0 Chemical species present in solution at point: 1) Before any addition of base – Large quantities of H3O+ and Cl- ions. Since strong acids dissociate 100%, there are large quantities of H3O+ and Cl- ions in solution. The H3O+ keeps the pH well below 7.0 2) After addition of NaOH – Large quantities of H3O+ still, Cl-, and increasing concentrations of Na+ ions. The OH- from the NaOH will be neutralized by the excess H3O+ present to form water, so there will not be any significant quantity of OH- before the equivalence point. The pH is still very low. 3) At equivalence – Large quantities of Na+ and Cl- ions. The pH is neutral (7.0) since there are insignificant amounts of both H3O+ and OH- (both equal 1.0 x 10-7 M). 4) After equivalence – Large quantities of Na+, Cl- and OH- ions. Now the OH- is in excess and makes the pH of the solution well above 7.0. pH verification: On the curve on the slide, a table shows exactly what pH is being measure by the pH probe at any give time during the titration. 1) After addition of 30.00 ml of 0.10 M NaOH, the pH read by the pH probe is 1.85. Is it possible to verify this based on the quantities of chemical species present? This problem is to be treated exactly like the following neutralization question: 30.00 ml of 0.10 M NaOH has just been added to 40.00 ml of 0.10 M HCl. What is the pH of the final solution? Since neutralization reactions are irreversible, we require the use of an IRF table based on moles of reactant. This goes to show that at this point in the titration, there is still an excess of 0.001 mol HCl(aq), which means that there is 0.001 mol H3O+ as well. These moles of H3O+ are in a total volume of 0.07000 L, so we can calculate the concentration. [H3O+] = 0.01428 M pH = 1.85. The pH probe reading is verified based on our calculations! 2) Verify using calculations the pH after a total addition of 60.00 ml of NaOH. To tackle this problem, do your calculation from scratch. Start fresh, disregarding any calculations you may have done earlier. You really want to treat the calculation as if this was asked: 60.00 ml of 0.10 M NaOH has just been added to 40.00 ml of 0.10 M HCl. What is the pH of the final solution? You can see that after the equivalence point, all HCl has been consumed, and now the NaOH is in excess. 0.002 mol of NaOH means that there are 0.002 mol OH- in the new solution volume of 0.1000 L. [OH-] = 0.02 M. pOH = 1.7 so pH = 12.3
- Characteristics of curve: Usually a small rise before flattens out into buffer region. Vertical portion at equivalence point is shorter (a smaller “S”). pH at equivalence is > 7.0 Chemical species present in solution at point: 1) Before any addition of base – Large quantities of HA and small quantities of H3O+ and A-. Weak acids dissociate very little so most of the acid is not dissociated. The small quantities of H3O+ generated is enough to make the pH < 7.0. 2) After addition of NaOH – Concentration of Na+ ions increases. Before half equivalence – [HA] > [A-]. H3O+ is present in small quantities, At half equivalence – [HA] = [A-]. When this happens the [H3O+] = Ka, which means the pH = pKa. After half equivalence – [A-] > [HA] 3) At equivalence – Large quantities of Na+ and A- ions. The A- ion is a base, and will react with water to produce small quantities of HA and OH-. The quantity of OH- produced is enough to increase the pH above 7.0. 4) After equivalence – Large quantities of Na+, A- and OH- ions. Now the OH- is in excess and makes the pH of the solution well above 7.0. pH verification: For the example in the slide, the titration is of propionic acid, CH3CH2COOH, or simply HPr. The Ka for the acid is 1.29 x 10-5 (which makes the Kb for the conjugate base 7.76 x 10-10) At equivalence, when 40.00 ml of NaOH has been added, why is the pH at equivalence above 7.0? At equivalence, the same moles of acid and base have reacted in a neutralization reaction. The Pr- produced from the neutralization reaction quickly reacts with water reversibly, creating an equilibrium. [Pr-] = 0.004 mol/0.08000 L = 0.0500 M Since Kb is so small, x will be small such that 0.05 – x ~ 0.05. Solving for x gives 6.63 x 10-6 M = [OH-] in the solution. pOH = 5.2 so pH = 8.8. The pH is greater than 7.0 at equivalence during the titration of a weak acid with strong base because of the presence of large quantities of conjugate base in solution.
- Take H2SO3 for example. It takes 2 molar equivalents of NaOH to fully neutralize this acid. H2SO3(aq) + 2 NaOH(aq) 2 Na+(aq) + SO32-(aq) + 2 H2O(l) So if 2 moles of NaOH was added to 1 mole of H2SO3 ALL AT ONCE, the products would be as shown in the right side of the equation. However, if only 1 mole of NaOH was slowly added to the acid first, ONLY the first H would be removed from the acid. H2SO3(aq) + 1 NaOH(aq) Na+(aq) + HSO3-(aq) + H2O(l) After adding another mole of NaOH, would the second H be removed. HSO3-(aq) + 1 NaOH(aq) Na+(aq) + SO32-(aq) + H2O(l)
- This is how the titration curve would look if the NaOH was slowly dripped into the H2SO3 acid. Notice that there are TWO equivalence points/humps. Before 40.00 ml of NaOH, only the first H is being neutralized. Right at the moment 40.00 ml of NaOH is added, the acid is all HSO3-. After adding another 40.00 ml NaOH, it all becomes SO32-. In this course, it is only important to understand the titration of polyprotic acids qualitatively. We will not be doing any pH calculations involving polyprotic acids.
- Most of the “bases” we’ve encountered in this course are classical bases which are ionic compounds: NaOH(s), KOH(s), Ba(OH)2(s). These compounds dissociate to form OH- ion in solution. Molecular bases (covalent/molecular compounds) have an N atom in the structure. N has a lone pair which can be used as a proton acceptor.
- Last topic: Adding slightly soluble (or insoluble) ionic compounds to water and stirring would result in a cloudy solution since the solid doesn’t fully dissolve. Question: the Ksp for CaSO4(s) is 4.93 x 10-5. If CaSO4 was added into a beaker of water and stirred, what would be the concentration of ions? According to the equilibrium reaction: CaSO4(s) Ca2+(aq) + SO42-(aq) I constant 0 0 R -x +x +x E constant x x The math is simple: since Ksp = [Ca2+] [SO42-], we essentially take the square root of Ksp to solve for the ion concentrations. At equilibrium, the concentration of Ca2+ and SO42- is 0.007 M.
- Common ion effect question: Would solid CaSO4(s) be more or less soluble in a solution of 0.01 M CaCl2(aq) compared to pure water? CaSO4(s) is already not completely soluble in water such that at equilibrium, the concentrations of the Ca2+ and SO42- are low. However, in a solution of CaCl2(aq), there are already high concentrations of Ca2+. If CaSO4(s) is added, the only way for Ksp to be maintained is if the reaction shifts to the left to produce the solid. Therefore, the solubility of CaSO4(s) is greatly reduced in a solution of CaCl2(aq). Effect of pH: CaCO3(s) is very insoluble and would be marked as an “I” on the solubility table. This compound has a Ksp of 6.0 x 10-9. If we added CaCO3(s) into water and stirred, very little of it would dissociate to form ions. Even so, the dissolving of this compound can be expressed by an equation: CaCO3(s) Ca2+(aq) + CO32-(aq) If we tried dissolving this compound in a solution of low pH, the H3O+ in solution would react with the carbonate to produce CO2(g) (bubbles away), and water. Since the CO3-(aq) ion is continuously being removed from the solution, the reaction would shift to the right to produce more. If enough H3O+ was present in solution, eventually all the CaCO3 would disappear. The solubility of this “insoluble” compound has increased greatly due to the low pH. Another example: Ba(OH)2(s) has a Ksp of 5.0 x 10-3 (slightly soluble ionic compound) Ba(OH)2(s) Ba2+(aq) + 2 OH-(aq) This compound would be more soluble in low pH solutions compared to water since the H3O+ would react with the hydroxide to produce water. This would shift the reaction to the right. This compound would be less soluble in high pH solutions compared to water since at low pH, hydroxide ion would already be present at high concentrations. The common ion effect would shift the reaction to the left, reducing the solubility. Questions: 1. Ionic CompoundKspA1.2B3.0 x 10-8C6.5 x 10-4 Rank the ionic compounds in order of increasing solubility in water. 2. Only 0.185 g of calcium hydroxide can dissolve in 100 ml of water and still remain clear (no solid in solution). Would more or less of it dissolve in a solution of: a) 0.10 M sodium hydroxide b) 0.10 M calcium nitrate c) 0.10 M sodium chloride 3. Barium hydroxide is a slightly soluble ionic compound. List 3 different solutions that would reduce its solubility compared to that of pure water. Answers: 1. B, C, A 2 .a) less b) less c) no change 3. barium chloride, sodium hydroxide, barium nitrate