Heterocyclic Ppt
- 55. Polymerization Reaction under acidic condition
Notes de l'éditeur
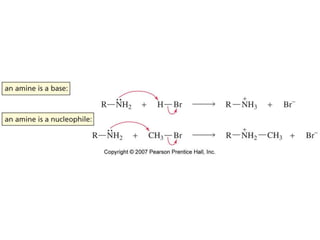
- Figure: 20-00-01UN Title: Amine as a base and nucleophile. Caption: The lone-pair electrons of the nitrogen atom cause amines to react as bases, sharing the lone pair with a proton, and as nucleophiles, sharing the lone pair with an atom other than a proton.
- Figure: 20-00-02UN Title: Classification and nomenclature of amines. Caption: Amines are classified by the number of carbons directly attached to the nitrogen. For the IUPAC names, change the "e" of the alkane to "amine." For common names, list each alkyl group followed by "amine."
- Figure: 20-00-03UN Title: Cyclic amines. Caption: The nitrogen is position one in the cyclic amines.
- Figure: 20-00-04UN Title: Heterocyclic compounds. Caption: The heteroatom has the lowest possible number.
- Figure: 20-00-05UN Title: Heterocyclic compounds. Caption: Position one is an atom that is not carbon.
- Figure: 20-00-13UN Title: The p K a values of ammonium ion, anilinium ion, and an amine. Caption: The greater acidity of anilinium ions compared with ammonium ions is due to the greater stability of the conjugate bases of the anilinium ion as a result of electron delocalization.
- Figure: 20-00-14UN Title: The p K a values of the ammonium ions of pyrrolidine, piperidine, morpholine, N -methylpyrrolidine, and quinuclidine. Caption: Saturated amine heterocycles containing five or more atoms have physical and chemical properties typical of acyclic amines.
- Figure: 20-00-16UN Title: Ethyl bromide reacts with methylamine to yield ethylmethylammonium bromide, which loses hydrogen bromide to give ethylmethylamine. Caption: The lone pair on the nitrogen of an amine causes it to be nucleophilic as well as basic.
- Figure: 20-00-17UN Title: Propanoyl chloride reacts with methylamine to yield N -methylpropanamide and methylammonium chloride. Caption: The amine attacks the carbonyl carbon.
- Figure: 20-00-18UN Title: Aldehydes and ketones react with primary amines to form imines and with secondary amines to form enamines. Caption: These reactions are examples of nucleophilic-elimination reactions.
- Figure: 20-00-19UN Title: Amines react with , -unsaturated aldehydes and ketones to result in a conjugate addition product. Caption: The amine group adds to the -carbon of the , -unsaturated aldehyde.
- Figure: 20-00-20UN Title: Primary arylamines react with nitrous acid to form arenediazonium salts. Caption: The diazonium group can be replaced with a wide variety of nucleophiles.
- Figure: 20-00-25UN Title: Hofmann elimination reaction. Caption: The reaction of a quaternary ammonium ion with hydroxide ion is known as a Hofmann elimination reaction.
- Figure: 20-00-27UN Title: Mechanism of the Hofmann elimination. Caption: Base abstracts the -hydrogen from the amine. A double bond is formed and the tertiary amine is expelled.
- Figure: 20-00-28UN Title: Hofmann elimination. Caption: The Hofmann elimination yields 1-pentene, 2-pentene, and trimethylamine.
- Figure: 20-00-29UN Title: Hofmann elimination. Caption: The Hofmann elimination of the quaternary amine yields isobutyldimethylamine and propene.
- Figure: 20-00-30UN Title: Removal of -hydrogens on alkyl halides versus amines. Caption: For amines, a carbanion-like transition state is formed. The carbon with the most hydrogens is the most stable.
- Figure: 20-00-35UN Title: Quaternary ammonium halides cannot undergo a Hofmann elimination. A quaternary ammonium halide can be converted into a quaternary ammonium hydroxide by treatment with silver oxide and water. Caption: If the halide is removed with hydroxide ion, the Hofmann elimination can occur.
- Figure: 20-00-36UN Title: Propylamine is treated with excess methyl iodide to yield trimethylpropylammonium iodide. Caption: The reaction of an amine with sufficient methyl iodide to convert the amine into a quaternary ammonium iodide is called exhaustive methylation.
- Figure: 20-00-38UNP09a Title: Give the synthesis, I. Caption: To prepare an alkene from an amine, an elimination reaction needs to occur.
- Figure: 20-00-41UNP09 sol Title: Give the synthesis, II. Caption: The amine reacts with excess methyl iodide to form a quaternary ammmonium iodide. The quaternary ammonium iodide is converted to a quaternary ammonium hydroxide. Heat is required for the elimination step.
- Figure: 20-00-42UN Title: Since the cyanide ion is soluble in one phase and the alkyl halide is soluble in another phase, how can the reaction occur? Caption: The two solutions are immiscible.
- Figure: 20-00-43UN Title: 1-Bromohexane reacts with sodium cyanide to yield heptanenitrile. Caption: A phase-transfer catalyst is used so that the two compounds can react with each other.
- Figure: 20-00-44UN Title: Phase-transfer catalysts. Caption: Quaternary ammonium salts are the most common phase-transfer catalysts.
- Figure: 20-00-45UN Title: Phase-transfer catalysis. Caption: The phase-transfer catalyst shuttles back and forth between the phases carrying the nucleophile from the aqueous phase to the organic phase to react with the electrophile, and then carrying the leaving group from the organic phase back to the aqueous phase where it is released.
- Figure: 20-00-46UN Title: Oxidation reactions of primary amines. Caption: A primary amine is oxidized to a hydroxylamine. A hydroxylamine is oxidized to a nitroso compound, which is oxidized to a nitro compound.
- Figure: 20-00-47UN Title: Oxidation reactions of secondary and tertiary amines. Caption: Secondary amines are oxidized to secondary hydroxylamines. Tertiary amines are oxidized to tertiary amine oxides.
- Figure: 20-00-48UN Title: Cope elimination reaction. Caption: Tertiary amine oxides undergo elimination in the presence of heat to give hydroxylamines and alkenes.
- Figure: 20-00-49UN Title: Mechanism of the Cope elimination reaction. Caption: The oxygen abstracts the hydrogen. A double bond is formed, with expulsion of the amine group.
- Figure: 20-00-50UN Title: Cope elimination. Caption: The Cope elimination of a tertiary amine oxide yields an alkene and a hydroxylamine.
- Figure: 20-00-55UN Title: Reaction of amines with alkyl halides. Caption: Amines undergo S N 2 reactions with alkyl halides.
- Figure: 20-00-56UN Title: Gabriel synthesis. Caption: Phthalimide reacts with an alkyl halide to yield a primary amine.
- Figure: 20-00-57UN Title: Butyl bromide reacts with azide ion, followed by hydrogenation, to give butylamine. Caption: The reaction is an S N 2 reaction.
- Figure: 20-00-58UN Title: Butyl bromide reacts with sodium cyanide to give pentanenitrile, which is hydrogenated to give pentylamine. Caption: The reaction is an S N 2 reaction.
- Figure: 20-00-59UN Title: Reduction of nitro groups to give amines. Caption: Nitroethane is reduced to ethylamine. Nitrobenzene is reduced to aniline.
- Figure: 20-00-60UN Title: Amides are reduced to amines with lithium aluminum hydride. Caption: Primary amides are reduced to primary amines with lithium aluminum hydride. Secondary amides are reduced to secondary amines with lithium aluminum hydride. Tertiary amides are reduced to tertiary amines with lithium aluminum hydride.
- Figure: 20-00-63UN Title: Pyrrole, furan, and thiophene. Caption: Pyrrole, furan, and thiophene are five-membered heterocyclic compounds.
- Figure: 20-00-64UN Title: Orbital structures of pyrrole and furan. Caption: Pyrrole and furan are aromatic because a lone pair of electrons on their heteroatoms delocalizes from a p orbital on the heteroatom into the ring, creating an aromatic complement of six pi ring electrons.
- Figure: 20-00-65UN Title: Resonance contributors of pyrrole. Caption: The nitrogen donates the electrons onto the five-membered ring.
- Figure: 20-00-66UN Title: Electrostatic potential maps of pyrrolidine and pyrrole. Caption: The nitrogen atom in pyrrolidine is red because the lone pair electrons in pyrrolidine are localized on the nitrogen. Since pyrrole is aromatic and its nitrogen's lone pair is delocalized, a buildup of electron density on nitrogen is not observed in pyrrole's potential map.
- Figure: 20-00-67UN Title: Relative resonance energies of some aromatic compounds. Caption: The resonance energy is the least stable for the compound with the most electronegative atom.
- Figure: 20-00-68UN Title: Mechanism for electrophilic aromatic substitution for pyrrole. Caption: The double bond attacks the electrophile. The carbocation is formed at position 3. Base removes the extra proton.
- Figure: 20-00-69UN Title: Furan is brominated to give 2-bromofuran. 2-Methylpyrrole is nitrated to yield 2-methyl-5-nitropyrrole. Caption: Furan and pyrrole undergo electrophilic aromatic substitution reactions.
- Figure: 20-00CO Title: Electrostatic potential maps of pyrrolidine, pyrrole, furan, and thiophene. Caption: Pyrrole, furan, and thiophene are aromatic.
- Figure: 20.1 Title: Figure 20.1. Structures of the intermediates that can be formed from the reaction of an electrophile with pyrrole at C-2 and C-3. Caption: Pyrrole undergoes electrophilic substitution preferentially at carbon 2.
- Figure: 20-01-01UN Title: 2,5-Dimethylfuran is brominated to give 3-bromo-2,5-dimethylfuran. Caption: If both positions adjacent to the heteroatom are occupied, electrophilic aromatic substitution occurs on C-3.
- Figure: 20-01-02UN Title: Relative reactivities toward electrophilic aromatic substitution. Caption: Pyrrole, furan, and thiophene are all more reactive than benzene toward electrophilic aromatic substitution.
- Figure: 20-01-03UN Title: Electrostatic potential maps of pyrrole, furan, and thiophene. Caption: From the potential maps it can be seen that the pyrrole ring is more electron rich than the furan ring, which is in turn more electron rich than the thiophene ring. Pyrrole's nitrogen donates its lone pair into the ring more readily than furan's oxygen due to electronegativity differences, and furan's oxygen donates electrons into the ring more readily than thiophene's sulfur due to poor overlap between sulfur and carbon in the thiophene pi system.
- Figure: 20-01-04UN Title: Benzene is acylated to yield acetophenone. Thiophene is acylated to give 2-acetylthiophene. Caption: If the aromatic compound is more reactive, than a weaker Lewis acid is needed.
- Figure: 20-01-05UN Title: Furan is acylated to give 2-acetylfuran. Pyrrole is acylated to give 2-acetylpyrrole. Caption: If the aromatic compound is more reactive, than a weaker Lewis acid is needed.
- Figure: 20-01-06UN Title: Pyrrole is protonated on carbon-2. Caption: Proton is an electrophile.
- Figure: 20-01-07UN Title: Pyrrole polymerizes in acidic solution. Caption: Pyrrole is unstable in strongly acidic solutions.
- Figure: 20-01-08UN Title: The p K a values of pyrrole and pyrrolidine. Caption: The partial positive charge on the nitrogen contributes to pyrrole’s increased acidity.
- Figure: 20.1 Title: Table 20.1. The p K a values of several nitrogen heterocycles. Caption: The aromatic amine is more acidic that the nonaromatic equivalent.
- Figure: 20-01-11UN Title: Orbital structure of pyridine. Caption: The lone-pair electrons on pyridine's nitrogen are held in a p orbital perpendicular to the pi system, so they do not delocalize into the ring, and are available for bonding to electrophiles.
- Figure: 20-01-12UN Title: The p K a values of pyridinium ion and piperidinium ion. Caption: The pyridinium ion is a stronger acid than the piperidinium ion.
- Figure: 20-01-13UN Title: Pyridine undergoes reactions characteristic of tertiary amines. Caption: Pyridine reacts with methyl iodide to form N -methylpyridinium iodide. Pyridine reacts with hydrogen peroxide to yield pyridine- N -oxide.
- Figure: 20-01-14UNP16 Sol Title: Will an amide be formed from the reaction of an acyl halide with an aqueous solution of pyridine? Caption: An amide will not be formed because the positively charged nitrogen causes pyridine to be an excellent leaving group.
- Figure: 20-01-15UN Title: Resonance contributors of pyridine. Caption: Pyridine is aromatic.
- Figure: 20-01-16UN Title: Electrostatic potential maps of benzene and pyridine. Caption: Since pyridine does not donate its lone-pair electrons into the ring, the pyridine ring does not have more electron density than the benzene ring. In fact, as the potential maps show, pyridine has less electron density in the ring than benzene because nitrogen is more electronegative than carbon, and pyridine's nitrogen sucks some of the electron density out of the ring.
- Figure: 20-01-17UN Title: Dipole moment of pyridine. Caption: The dipole moment of pyridine is 1.57 D.
- Figure: 20-01-18UN Title: Mechanism for electrophilic aromatic substitution. Caption: The double bond in pyridine attacks the electrophile to form the carbocation intermediate. Base removes the proton and the aromatic ring is regenerated.
- Figure: 20.2 Title: Figure 20.2. Structures of the intermediates that were formed from the reaction of an electrophile with pyridine. Caption: Electrophilic aromatic substitution occurs mainly at carbon-3 of pyridine, because the attack at these positions leads to the most stable intermediates.
- Figure: 20-02-01UN Title: Relative reactivity in nucleophilic aromatic substitution. Caption: Benzene is the most reactive.
- Figure: 20-02-02UN Title: Electrophilic aromatic substitution reactions of pyridine. Caption: Pyridine is brominated to give 3-bromopyridine. Pyridine is sulfonated to give pyridine-3-sulfonic acid. Pyridine is nitrated to give 3-nitropyridine.
- Figure: 20-02-03UN Title: Pyridine does not undergo Friedel-Crafts reactions. Caption: Pyridine is more deactivated than benzene and does not undergo Friedel-Crafts reactions.
- Figure: 20-02-05UN Title: Mechanism for the nucleophilic aromatic substitution of pyridine. Caption: The nucleophile attacks the carbon bearing the leaving group. The leaving group departs.
- Figure: 20.3 Title: Figure 20.3. Structures of the intermediates that can be formed from the reaction of a nucleophile with pyridine. Caption: Nucleophilic aromatic substitution occurs mainly at carbon-2 and carbon-4 of pyridine, because the attack at these positions leads to the most stable intermediates.
- Figure: 20-03-01UN Title: If the leaving groups at carbon-2 and carbon-4 are different, the incoming nucleophile will preferentially substitute for the weaker base. Caption: 4-Bromo-2-methoxypyridine reacts with amide ion to form 4-amino-2-methoxypyridine. 2-Chloro-4-methylpyridine reacts with methoxide ion to form 2-methoxy-4-methylpyridine.
- Figure: 20-03-04UN Title: Substituted pyridines undergo side-chain reactions. Caption: Alkyl-substituted pyridines can be brominated and oxidized.
- Figure: 20-03-05UN Title: Aminopyridines are diazotized. Caption: 2-Aminopyridine is diazotized, then hydrolyzed, to form -pyridone. 4-Aminopyridine is diazotized, then hydrolyzed, to form -pyridone.
- Figure: 20-03-06UN Title: Resonance structures of the anion of 4-methylpyridine. Caption: The electron-withdrawing nitrogen causes the -hydrogens of the alkyl groups attached to the 2- and 4-positions of the pyridine ring to have about the same acidity as the -hydrogens of ketones.
- Figure: 20-03-07UN Title: Reactions of the anions of alkyl-substituted pyridines. Caption: 4-Methylpyridine can undergo an aldol condensation. 2,3-Dimethylpyridine can be alkylated.
- Figure: 20-03-09UN Title: The p K a values of quinoline and isoquinoline. Caption: Quinoline and isoquinoline are benzopyridines.
- Figure: 20-03-10UN Title: Proline, tryptophan, and histidine. Caption: Proline, tryptophan, and histidine are amino acids with heterocyclic rings.
- Figure: 20-03-11UN Title: Orbital structure of imidazole. Caption: The lone pair on the imidazole nitrogen attached to hydrogen is not basic or nucleophilic because it is delocalized into the ring, whereas the lone pair on the nitrogen which is not attached to hydrogen is nucleophilic and basic because it does not delocalize into the ring.
- Figure: 20-03-12UN Title: Resonance contributors of imidazole. Caption: The resonance energy of imidazole is less than the resonance energy of benzene.
- Figure: 20-03-13UN Title: The p K a value of protonated imidazole. Caption: Imidazole exists in protonated and nonprotonated forms at a pH of 7.3.
- Figure: 20-03-14UN Title: The p K a value of imidazole. Caption: Imidazole exists in protonated and nonprotonated forms at a pH of 7.3.
- Figure: 20-03-15UN Title: Protonated imidazole and imidazole anion. Caption: Both protonated imidazole and imidazole anion have two equivalent resonance contributors.
- Figure: 20-03-17UN Title: Purine, pyrimidine, adenine, guanine, cytosine, uracil, and thymine. Caption: Adenine and guanine have a purine structure. Cytosine, uracil, and thymine have a pyrimidine structure.
- Figure: 20-03-18UN Title: Porphyrins. Caption: A porphyrin ring system has four pyrrole rings joined by one-carbon bridges.
- Figure: 20-01-10UN Title: Indole, benzofuran, and benzothiophene. Caption: Indole, benzofuran, and benzothiophene contain a five-membered ring fused onto a benzene ring.