10b motor system voluntary control
- 2. Control of voluntary movement Idea Association cortex Premotor + Motor cortex Basal Ganglia Lateral cerebellum Movement Intermediate Cerebellum Execution Planning
- 3. Modulation of Movement by the Basal Ganglia
- 4. Motor components of the human basal ganglia
- 5. Anatomical Organization of Basal Ganglia Input
- 6. Output of Basal Ganglia
- 7. The somatotopic organization of the basal ganglia-thalamocortical motor circuit
- 11. Functional organization of cerebellum
- 12. Input of Cerebellum
- 15. Neocerebellum
- 16. The spinocerebellum contains two somatotopic neural maps of the body
- 20. Motor Cortex
- 21. Functional Organization of the Primary Motor Cortex
- 22. The major inputs to the motor cortex in monkeys
- 25. Convergence of Motor Control on the Anterior Motor Neuron
- 26. Experimental apparatus developed to record the activity of single neurons in awake primates trained to perform specific movements : Ed Evarts 1960
- 27. Direct corticospinal control of motor neurons is necessary for fine control of the digits
- 28. Directional tuning of an upper motor neuron in the primary motor cortex
- 29. Activity in Individual Neurons of the Primary Motor Cortex Is Related to Muscle Force and Direction of Movement
- 30. Spike Triggered Averaging 1970
- 31. Motor Cortical Cell Firing with Force Generated
- 32. Corticomotoneuronal (CM) cell is active depends on the motor task
- 33. Different areas of cortex are activated during simple, complex, and imagined sequences of finger movements (Xenon PET)
- 34. Cell activity in the motor cortex depends on whether a sequence of movements is guided by visual cues or by prior training
- 35. A set-related neuron in the dorsal premotor area becomes active while the monkey prepares to make a movement to the left
- 36. The visuomotor transformations required for reaching and grasping involve two different pathways
- 37. Individual neurons in the ventral premotor area fire during specific hand actions only
- 38. A. Activity in the neuron as the monkey observes another monkey make a precision group. B. Activity in the same neuron as the monkey observes the human experimenter make the precision grip. C. Activity in the same neuron as the monkey itself performs a precision grip. (From Rizzolotti et al 1996.) Mirror Neurons
- 40. MIRROR SYSTEMS CAN INFER HIDDEN GOALS:
- 45. Grasping intentions with mirror neurons Iacoboni and Dapretto Redgrave Nature Reviews Neuroscience 7 , 942 – 951 (December 2006) | doi:10.1038/ nrn2024
Notes de l'éditeur
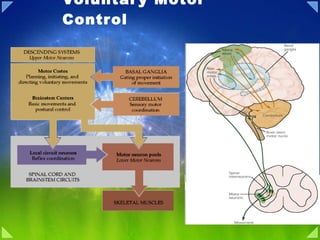
- Figure 16.1. Overall organization of neural structures involved in the control of movement. Four systems—local spinal cord and brainstem circuits, descending modulatory pathways, the basal ganglia, and the cerebellum—make essential and distinct contributions to motor control. Lower Motor Neuron Circuits and Motor Control Overview Skeletal (striated) muscle contraction is initiated by “lower” motor neurons in the spinal cord and brainstem. The cell bodies of the lower neurons are located in the ventral horn of the spinal cord gray matter and in the motor nuclei of the cranial nerves in the brainstem. These neurons (also called α motor neurons) send axons directly to skeletal muscles via the ventral roots and spinal peripheral nerves, or via cranial nerves in the case of the brainstem nuclei. The spatial and temporal patterns of activation of lower motor neurons are determined primarily by local circuits located within the spinal cord and brainstem. Descending pathways comprising the axons of “upper” motor neurons modulate the activity of lower motor neurons by influencing this local circuitry. The cell bodies of upper motor neurons are located either in the cortex or in brainstem centers, such as the vestibular nucleus, the superior colliculus, and the reticular formation. The axons of the upper motor neurons typically contact the local circuit neurons in the brainstem and spinal cord, which, via relatively short axons, contact in turn the appropriate combinations of lower motor neurons. The local circuit neurons also receive direct input from sensory neurons, thus mediating important sensory motor reflexes that operate at the level of the brainstem and spinal cord. Lower motor neurons, therefore, are the final common pathway for transmitting neural information from a variety of sources to the skeletal muscles. Neural Centers Responsible for Movement The neural circuits responsible for the control of movement can be divided into four distinct but highly interactive subsystems, each of which makes a unique contribution to motor control (Figure 16.1). The first of these subsystems is the local circuitry within the gray matter of the spinal cord and the analogous circuitry in the brainstem. The relevant cells include the lower motor neurons (which send their axons out of the brainstem and spinal cord to innervate the skeletal muscles of the head and body, respectively) and the local circuit neurons (which are the major source of synaptic input to the lower motor neurons). All commands for movement, whether reflexive or voluntary, are ultimately conveyed to the muscles by the activity of the lower motor neurons; thus they comprise, in the words of the great British neurophysiologist Charles Sherrington, the “final common path” for movement. The local circuit neurons receive sensory inputs as well as descending projections from higher centers. Thus, the circuits they form provide much of the coordination between different muscle groups that is essential for organized movement. Even after the spinal cord is disconnected from the brain in an experimental animal such as a cat, appropriate stimulation of local spinal circuits elicits involuntary but highly coordinated movements of the four limbs that resemble walking. The second motor subsystem consists of neurons whose cell bodies lie in the brainstem or cerebral cortex. The axons of these higher-order or upper motorneurons descend to synapse with the local circuit neurons or, more rarely, with the lower motor neurons directly. The upper motor neuron pathways that arise in the cortex are essential for the initiation of voluntary movements and for complex temporal sequences of movement. In particular, descending projections from cortical areas in the frontal lobe, including Brodmann's area 4 (the primary motor cortex), the lateral part of area 6 (the lateral premotor cortex ), and the medial part of area 6 (the medial premotor cortex ) are essential for planning, initiating, and directing temporal sequences of voluntary movements. Upper motor neurons originating in the brainstem are responsible for regulating muscle tone and for orienting the eyes, head, and body with respect to vestibular, somatic, auditory, and visual sensory information. Their contributions are thus critical for basic navigational movements of the body, and in the control of posture. The third and fourth subsystems are structures (or groups of structures) that have no direct access to either the local circuit neurons or the lower motorneurons; instead, they control movement by regulating the activity of the upper motor neurons. The third and larger of these subsystems, the cerebellum, is located on the dorsal surface of the pons (see Chapter 1). The cerebellum acts via its efferent pathways to the upper motor neurons as a servomechanism, detecting the difference, or “motor error,” between an intended movement and the movement actually performed (see Chapter 19). The cerebellum uses this information about discrepancies to mediate both real-time and long-term reductions in these motor errors (the latter being a form of motor learning). As might be expected from this account, patients with cerebellar damage exhibit persistent errors in movement. The fourth subsystem, embedded in the depths of the forebrain, consists of a group of structures collectively referred to as the basal ganglia (see Chapter 1). The basal ganglia suppress unwanted movements and prepare (or “prime”) upper motor neuron circuits for the initiation of movements. The problems associated with disorders of basal ganglia, such as Parkinson's disease and Huntington's disease, attest to the importance of this complex in the initiation of voluntary movements (see Chapter 18). Despite much effort, the sequence of events that leads from thought to movement is still poorly understood. The picture is clearest, however, at the level ofcontrol of the muscles themselves. It therefore makes sense to begin an account of motor behavior by considering the anatomical and physiological relationships between lower motor neurons and the muscle fibers they innervate The Motor Systems Are Organized Hierarchicaly The Spinal Cord, Brain Stem, and Forebrain Contain Successively More Complex Motor Circuits The motor systems can perform so many different motor tasks—reflex, rhythmic, and voluntary—with speed and accuracy because of two features of their functional organization. First, the processing of sensory inputs and commands to motor neurons and muscles is distributed in hierarchically interconnected areas of the spinal cord, brain stem, and forebrain. Each level has circuits that can, through their input and output connections, organize or regulate complex motor responses. Second, sensory information relating to movement is processed in different systems that operate in parallel. The hierarchical organization of the motor systems is illustrated in Figure 33-12. The spinal cord is the lowest level of this hierarchical organization. It contains the neuronal circuits that mediate a variety of reflexes and rhythmic automatisms such as locomotion and scratching. Similar circuits governing reflex movements of the face and mouth are located in the brain stem. The simplest neural circuit is monosynaptic; it includes only the primary sensory neuron and the motor neuron. However, most reflexes are mediated by polysynaptic circuits, where one or more interneurons are interposed between the primary sensory neuron and the motor neuron. Interneurons and motor neurons also receive input from axons descending from higher centers. These supraspinal signals can modify reflex responses to peripheral stimuli by facilitating or inhibiting different populations of interneurons. They also coordinate motor actions through these interneurons. For example, when we flex a joint the descending commands that drive the flexor muscle also inhibit the opposing extensor muscle through the same inhibitory interneuron that is activated during the stretch reflex. Nevertheless, all motor commands eventually converge on motor neurons, whose axons exit the spinal cord or brain stem to innervate skeletal muscles. Thus in Sherrington's words, motor neurons are the “final common pathway” for all motor action. The next level of the motor hierarchy is in the brain stem. Two systems of brain stem neurons, the medial and lateral, receive input from the cerebral cortex and subcortical nuclei and project to the spinal cord. The medial descending systems of the brain stem contribute to the control of posture by integrating visual, vestibular, and somatosensory information. The lateral descending systems control more distal limb muscles and are thus important for goal-directed movements, especially of the arm and hand. Other brain stem circuits control movements of the eyes and head. The cortex is the highest level of motor control. The primary motor cortex and several premotor areas project directly to the spinal cord through the corticospinal tract and also regulate motor tracts that originate in the brain stem. The premotor areas are important for coordinating and planning complex sequences of movement. They receive information from the posterior parietal and prefrontal association cortices (see Chapter 19) and project to the primary motor cortex as well as to the spinal cord. The variety of reflex circuits in the spinal cord and brain stem simplifies the instructions the cortex must send to lower levels. By facilitating some circuits and inhibiting others, higher levels can let sensory inputs at lower levels govern the temporal details of an evolving movement. The timing of activation of agonists and antagonist muscles is intrinsic to the spinal circuit and thus the descending signals themselves need not be timed as precisely. The patterns of coordination in spinal circuits are relatively stereotyped. A cat with its cervical cord transected can, if provided with body support, walk on a moving treadmill and bring its paw around an obstacle after hitting it. But the spinal cat cannot lift its forelimb before impact with an obstacle, as an intact animal does, because this movement requires control of the limbs using visual information. This anticipatory control, in turn, requires intervention by the motor cortex to suppress the oscillatory circuit that coordinates normal stepping. The Cerebellum and Basal Ganglia Influence Cortical and Brain Stem Motor Systems In addition to the three hierarchical levels—spinal cord, brain stem, and cortex—two other parts of the brain also regulate the planning and execution of movement. The cerebellum and basal ganglia provide feedback circuits that regulate cortical and brain stem motor areas: They receive inputs from various areas of cortex and project to motor areas of the cortex via the thalamus. The loop circuits of these two structures flow through separate regions of the thalamus and to different cortical areas. Likewise, the inputs to them from the cortex are also separate. The cerebellum and basal ganglia do not send significant output to the spinal cord, but they do act directly on motor neurons in the brain stem. Summary Four distinct but highly interactive motor subsystems—local circuits in the spinal cord and brainstem, descending upper motor neuron pathways that controlthese circuits, the basal ganglia, and the cerebellum—all make essential contributions to motor control. Alpha motor neurons located in the spinal cord and in the cranial nerve nuclei in the brainstem directly link the nervous system and muscles, with each motor neuron and its associated muscle fibers constituting a functional entity called the motor unit. Motor units vary in size, amount of tension produced, speed of contraction, and degree of fatigability. Graded increases in muscle tension are mediated by both the orderly recruitment of different types of motor units and an increase in motor neuron firing frequency. Local circuitry involving sensory inputs, local circuit neurons, and α and γ motor neurons are especially important in the reflexive control of muscle activity. The stretch reflex is a monosynaptic circuit with connections between sensory fibers arising from muscle spindles and the α motor neurons that innervate the same or synergistic muscles. Gamma motor neurons regulate the gain of the stretch reflex by adjusting the level of tension in the intrafusal muscle fibers of the muscle spindle. This mechanism sets the baseline level of activity in α motor neurons and helps to regulate muscle length and tone. Other reflex circuitsprovide feedback control of muscle tension and mediate essential functions such as the rapid withdrawal of limbs from painful stimuli. Much of the spatial coordination and timing of muscle activation required for complex rhythmic movements such as locomotion are provided by specialized local circuits called central pattern generators. Because of their essential role in all of these circuits, damage to lower motor neurons leads to paralysis of the associated muscle and to other changes, including the loss of reflex activity, the loss of muscle tone, and eventually muscle atrophy. Figure 17.7. The primary motor cortex and the premotor area in the human cerebral cortex as seen in lateral (A) and medial (B) views. The primary motor cortex is located in the precentral gyrus; the premotor area is more rostral. The Primary Motor Cortex: Upper Motor Neurons That Initiate Complex Voluntary Movements The upper motor neurons in the cerebral cortex reside in several adjacent and highly interconnected areas in the frontal lobe, which together mediate the planning and initiation of complex temporal sequences of voluntary movements. These cortical areas all receive regulatory input from the basal ganglia and cerebellum via relays in the ventrolateral thalamus (see Chapters 18 and 19), as well as inputs from the somatic sensory regions of the parietal lobe (seeChapter 9). Although the phrase “motor cortex” is sometimes used to refer to these frontal areas collectively, more commonly it is restricted to the primarymotor cortex, which is located in the precentral gyrus (Figure 17.7). The primary motor cortex can be distinguished from the adjacent “premotor” areas both cytoarchitectonically (it is area 4 in Brodmann's nomenclature) and by the low intensity of current necessary to elicit movements by electrical stimulation in this region. The low threshold for eliciting movements is an indicator of a relatively large and direct pathway from the primary area to the lower motorneurons of the brainstem and spinal cord. This section and the next focus on the organization and functions of the primary motor cortex and its descending pathways, whereas the subsequent section addresses the contributions of the adjacent premotor areas. The pyramidal cells of cortical layer V (also called Betz cells) are the upper motor neurons of the primary motor cortex. Their axons descend to the brainstem and spinal motor centers in the corticobulbar and corticospinal tracts, passing through the internal capsule of the forebrain to enter the cerebral peduncle at the base of the midbrain (Figure 17.8). They then run through the base of the pons, where they are scattered among the transverse pontine fibers and nuclei of the pontine gray matter, coalescing again on the ventral surface of the medulla where they form the medullary pyramids . The components of this upper motor neuron pathway that innervate cranial nerve nuclei, the reticular formation, and the red nucleus (that is, the corticobulbar tract) leave the pathway at the appropriate levels of the brainstem (see Figure 17.8 and Box A). At the caudal end of the medulla, most, but not all, of the axons in the pyramidal tract cross (or “decussate”) to enter the lateral columns of the spinal cord, where they form the lateral corticospinal tract . A smaller number of axons enters the spinal cord without crossing; these axons, which comprise the ventral corticospinal tract , terminate either ipsilaterally or contralaterally, after crossing in the midline (via spinal cord commissure). The ventral corticospinal pathway arises primarily from regions of the motor cortex that serve axial and proximal muscles (see Figure 17.6). The lateral corticospinal tract forms the direct pathway from the cortex to the spinal cord and terminates primarily in the lateral portions of the ventral horn and intermediate gray matter (see Figures 17.6 and 17.8). The indirect pathway to lower motor neurons in the spinal cord runs, as already described, from the motor cortex to two of the sources of upper motor neurons in the brainstem: the red nucleus and the reticular formation. In general, the axons to the reticular formation originate from the parts of the motor cortex that project to the medial region of the spinal cord gray matter, whereas the axons to the red nucleus arise from the parts of the motor cortex that project to the lateral region of the spinal cord gray matter (see Figure 17.6). Figure 38-1 Motor cortical areas are organized somato-topically. A. Brodmann's cytoarchitectural areas in monkeys and humans. B. Comparison of the somatotopic organization of the primary motor cortex in monkeys and humans. The sequence of representation of body parts is similar. The ankle control area is medial while the face, mouth, and mastication control areas are lateral. The face and fingers in the human motor cortex have much larger representations because of the greater degree of cortical control of these areas. (Left: from Woolsey 1958; right: adapted from Penfield and Rasmussen 1950.) C. Somatotopic organization of the medial and lateral motor cortex in the monkey, showing the arm and leg representations. (ArSi, arcuate sulcus, inferior limb; ArSs = arcuate sulcus, superior limb; CS = central sulcus; M1 = primary motor cortex; PMd = dorsal premotor area; PMv = ventral premotor area; PS = precentral sulcus; SGm = superior frontal gyrus, medial wall; SMA = supplementary motor area; pre-SMA = presupplementary motor area; SPcS = superior precentral sulcus.) (From Dum and Strick 1996.) An Overall View The goal of postural control is to orient body parts relative to one another and the external world without loss of balance. Posture must be controlled both while the body is still (static equilibrium) and during movement (dynamic equilibrium). In the dynamic states of natural behavior voluntary movement can perturb postural equilibrium, but knowledge of these potential perturbations is built into the motor program and used to offset their adverse effects ahead of the event by anticipatory (feed-forward) motor action. These anticipatory responses tend to be complex, involving many synergistic muscle groups. Anticipatory responses must be learned, but eventually they operate automatically, being triggered by specific intended movements. The postural system is also equipped with stereotyped response patterns that are rapidly corrected for unexpected perturbations. Some of these responses are innate, while others have to be acquired by motor learning that involves the cerebellum. These responses are characteristically driven by immediate feedback from visual, vestibular, and somatosensory information. In the past, posture might have been explained by the parallel action of involuntary reflexes controlled at relatively low levels of the nervous system. Today we recognize that postural control is complex and context-dependent and that all levels of the nervous system must be examined to account for this complexity. Voluntary Movement Is Organized in the Cortex The Primary Motor Cortex Controls Simple Features of Movement The discovery in 1870 that electrical stimulation of different parts of the frontal lobe produces movements of muscles on the opposite side of the body had a major impact on neurological thinking. In the early twentieth century electrical stimulation was used to identify the specific motor effects of discrete sites in the frontal lobe in different species—including primates and humans—and the resulting motor maps were correlated with anatomical and clinical observations on the effects of local lesions. The contralateral precentral gyrus (Brodmann's area 4), the region now called the primary motor cortex , proved to be the area in which the lowest-intensity stimulation elicited movements. At low intensities the effects of stimuli can be attributed to the activation of neurons near the electrode that are connected to the spinal cord either directly or via only a small number of synapses. The motor maps produced by these experiments show an orderly arrangement along the gyrus of control areas for the face, digits, hand, arm, trunk, leg, and foot. However, the fingers, hands, and face—which are used in tasks requiring the greatest precision and finest control—have disproportionately large representations in the motor areas of cortex (Figure 38-1), much as the inputs from regions of the body that have important roles in perception predominate in the sensory areas of the cortex. Consistent with the overall somatotopic organization, lesions in arm representation lead to degeneration of myelinated fibers in the cevical cord, while lesions in the leg representation produced degeneration extending all the way to the lumbar spinal cord. These axons arise from specialized large pyramidal neurons in lamina V named Betz cells after their discoverer
- Nevertheless, there is considerable evidence for the general motor control scheme shown in Figure 12-1 . Commands for voluntary movement originate in cortical association areas. The movements are planned in the cortex as well as in the basal ganglia and the lateral portions of the cerebellar hemispheres, as indicated by increased electrical activity before the movement. The basal ganglia and cerebellum both funnel information to the premotor and motor cortex by way of the thalamus. Motor commands from the motor cortex are relayed in large part via the corticospinal tracts to the spinal cord and the corresponding corticobulbar tracts to motor neurons in the brain stem. However, collaterals from these pathways and a few direct connections from the motor cortex end on brain stem nuclei, which also project to motor neurons in the brain stem and spinal cord. These pathways can also mediate voluntary movement. Movement sets up alterations in sensory input from the special senses and from muscles, tendons, joints, and the skin. This feedback information, which adjusts and smoothes movement, is relayed directly to the motor cortex and to the spinocerebellum. The spinocerebellum projects in turn to the brain stem. The main brain stem pathways that are concerned with posture and coordination are the rubrospinal, reticulospinal, tectospinal, and vestibulospinal tracts and corresponding projections to motor neurons in the brain stem.
- Figure 43-1 The relationships of the basal ganglia to the major components of the motor system. The basal ganglia and the cerebellum may be viewed as key elements in two parallel reentrant systems that receive input from and return their influences to the cerebral cortex through discrete and separate portions of the ventrolateral thalamus. They also influence the brain stem and, ultimately, spinal mechanisms. Modulation of Movement by the Basal Ganglia Overview As described in the preceding chapter, motor regions of the cortex and brainstem contain upper motor neurons that initiate movement by projecting to local circuit and lower motor neurons in the brainstem and spinal cord. This chapter and the next discuss two additional regions of the brain that are important in motor control: the basal ganglia and the cerebellum. In contrast to the components of the motor system that harbor upper motor neurons, the basal ganglia and cerebellum do not project directly to either the local circuit or lower motor neurons; instead, they influence movement by regulating the activity of upper motor neurons. The term "basal ganglia" refers to a large and functionally diverse set of nuclear structures that lie deep within the cerebral hemispheres. The subset of these nuclei relevant to motor function includes the caudate, putamen, and the globus pallidus. Two additional structures, the substantia nigra in the base of the midbrain and the subthalamic nucleus in the ventral thalamus, are closely associated with the motor functions of these basal ganglia nuclei and are included in the discussion. The motor components of the basal ganglia, together with the substantia nigra and the subthalamic nucleus, effectively make a subcortical loop that links most areas of the cortex with pools of upper motor neurons in the primary motor and premotor cortex and in the brainstem. The neurons in this loop respond in anticipation of and during movements, and their effects on upper motor neurons are required for the normal initiation of voluntary movements. When one of these components of the basal ganglia or associated structures is compromised, the patient cannot switch smoothly between commands that initiate a movement and those that terminate the movement. The disordered movements that result can be understood as a consequence of abnormal upper motor neuron activity in the absence of the supervisory control normally provided by the basal ganglia. The Basal Ganglia Mahlon R. DeLong THE BASAL GANGLIA CONSIST of four nuclei, portions of which play a major role in normal voluntary movement. Unlike most other components of the motor system, however, they do not have direct input or output connections with the spinal cord. These nuclei receive their primary input from the cerebral cortex and send their output to the brain stem and, via the thalamus, back to the prefrontal, premotor, and motor cortices. The motor functions of the basal ganglia are therefore mediated, in large part, by motor areas of the frontal cortex. Clinical observations first suggested that the basal ganglia are involved in the control of movement and the production of movement disorders. Postmortem examination of patients with Parkinson disease, Huntington disease, and hemiballismus revealed pathological changes in these subcortical nuclei. These diseases have three characteristic types of motor disturbances: (1) tremor and other involuntary movements; (2) changes in posture and muscle tone; and (3) poverty and slowness of movement without paralysis. Thus, disorders of the basal ganglia may result in either diminished movement (as in Parkinson disease) or excessive movement (as in Huntington disease). In addition to these disorders of movement, damage to the basal ganglia is associated with complex neuropsychiatric cognitive and behavioral disturbances, reflecting the wider role of these nuclei in the diverse functions of the frontal lobes. Primarily because of the prominence of movement abnormalities associated with damage to the basal ganglia, they were believed to be major components of a motor system, independent of the pyramidal (or corticospinal) motor system, the “extrapyramidal” motor system. Thus, two different motor syndromes were distinguished: the pyramidal tract syndrome , characterized by spasticity and paralysis, and the extrapyramidal syndrome , characterized by involuntary movements, muscular rigidity, and immobility without paralysis. There are several reasons why this simple classification is no longer satisfactory. First, we now know that, in addition to the basal ganglia and corticospinal systems, other parts of the brain participate in voluntary movement. Thus, disorders of the motor nuclei of the brain stem, red nucleus, and cerebellum also result in disturbances of movement. Second, the extrapyramidal and pyramidal systems are not truly independent but are extensively interconnected and cooperate in the control of movement. Indeed, the motor actions of the basal ganglia are mediated in large part through the supplementary, premotor, and motor cortices via the pyramidal system. Because they are so common, disorders of the basal ganglia have always been important in clinical neurology. Parkinson disease was the first disease of the nervous system to be identified as a molecular disease caused by a specific defect in transmitter metabolism. Therefore, in addition to providing important information about motor control, the study of diseased basal ganglia has provided a paradigm for studying the relationship of transmitters to disorders of mood, cognition, and nonmotor behavior, topics that will be considered in detail in Chapters 60 and 61. The use of a variety of anatomical, molecular, and neural imaging techniques as well as animal models of basal ganglia disorders has led to major advances in understanding the organization and function of the basal ganglia. These insights have, in turn, led to new pharmacologic and neurosurgical approaches to treatment of diseases of the basal ganglia. The Basal Ganglia Consist of Four Nuclei The basal ganglia consist of several interconnected subcortical nuclei with major projections to the cerebral cortex, thalamus, and certain brain stem nuclei. They receive major input from the cerebral cortex and thalamus and send their output back to the cortex (via the thalamus) and to the brain stem (Figure 43-1). Thus, the basal ganglia are major components of large cortical- subcortical reentrant circuits linking cortex and thalamus.
- Figure 18.1. Motor components of the human basal ganglia. (A) Basic circuits of the basal ganglia pathway: (+) and (-) denote excitory and inhibitory connections. (B) Idealized coronal section through the brain showing anatomical locations of structures involved in the basal ganglia pathway. Most of these structures are in the telencephalon, although the substantia nigra is in the midbrain and the thalamic and subthalamic nuclei are in the diencephalon. The ventral anterior and ventral lateral thalamic nuclei (VA/VL complex) are the targets of the basal ganglia, relaying the modulatory effects of the basal ganglia to upper motor neurons in the cortex. Projections to the Basal Ganglia The basal ganglia are divided into several functionally distinct groups of nuclei ( Figure 18.1 ). The first and larger of these groups is called the corpus striatum , which includes the caudate and putamen . These two subdivisions of the corpus striatum are the input zone of the basal ganglia, their neurons being the targets of most of the pathways that reach this complex from other parts of the brain ( Figure 18.2). The name (which means "striped body") is given because the axon fascicles that pass through the caudate and putamen give them a striped appearance when cut in cross section. The destination of the incoming axons from the cortex are onto the dendrites of a class of cells called medium spiny neurons in the corpus striatum ( Figure 18.3 ). The large dendritic trees of these neurons allow them to integrate inputs from a variety of cortical, thalamic, and brainstem structures. The axons arising in turn from the medium spiny neurons converge on neurons in the globus pallidus and the substantia nigra pars reticulata. The globus pallidus and substantia nigra pars reticulata are the main sources of output from the basal ganglia complex.
- Figure 18.2. Anatomical organization of the inputs to the basal ganglia. An idealized coronal section through the human brain, showing the projections from the cerebral cortex and the substantia nigra pars comparta to the caudate and putamen. Nearly all regions of the neocortex project directly to the corpus striatum, making the cerebral cortex the largest input to the basal ganglia by far. Indeed, the only cortical areas that do not project to the corpus striatum are the primary visual and primary auditory cortices ( Figure 18.4 ). Of those cortical areas that do innervate the striatum, the heaviest projections are from association areas in the frontal and parietal lobes, with substantial contributions from the temporal, insular, and cingulate cortices as well. All of these projections, referred to collectively as the corticostriatal pathway , travel through the internal capsule to reach the caudate and putamen directly (see Figure 18.2 ). Figure 18.3. Neurons and circuits of the basal ganglia. (A) Medium spiny neurons in the caudate and putamen. (B) Diagram showing convergent inputs onto a medium spiny neuron from cortical neurons, dopaminergic cells of the substantia nigra, and local circuit neurons. The primary output of the medium spiny cells is to the globus pallidus and to the substantia nigra pars reticulata.
- Figure 18.4. Regions of the cerebral cortex (shown in purple) that project to the caudate, putamen, and ventral striatum (see Box C) in both lateral (A) and medial (B) views. The caudate, putamen, and ventral striatum receive cortical projections primarily from the association areas of the frontal, parietal, and temporal lobes. The cortical inputs to the caudate and putamen are not equivalent, however, a fact that reflects functional differences between these two nuclei. The caudate nucleus receives cortical projections primarily from multimodal association cortices, and from motor areas in the frontal lobe that control eye movements. As the name implies, the association cortices do not process any one type of sensory information; rather, they receive inputs from a number of primary and secondary sensory cortices and associated thalamic nuclei (see Chapter 26). The putamen, on the other hand, receives input from the primary and secondary somatic sensory cortices in the parietal lobe, the secondary (extrastriate) visual cortices in the occipital and temporal lobes, the premotor and motor cortices in the frontal lobe, and the auditory association areas in the temporal lobe. The fact that different cortical areas project to different regions of the striatum implies that the corticostriatal pathway consists of multiple parallel pathways serving different functions. This interpretation is supported by the observation that the segregation is maintained in the structures that receive projections from the striatum, and in the pathways that project from the basal ganglia to other brain regions. Figure 18.5. Functional organization of the outputs from the basal ganglia. (A) Diagram of the targets of the basal ganglia, including the intermediate relay nuclei (the globus pallidus, internal and external segments, and the subthalamic nucleus), the superior colliculus, the thalamus, and the cerebral cortex. (B) An idealized coronal section through the human brain, showing the structures and pathways diagrammed in (A). There are other indications that the corpus striatum is functionally subdivided according to its inputs. For example, visual and somatic sensory cortical projections are topographically mapped within different regions of the putamen. Moreover, the cortical areas that are functionally interconnected at the level of the cortex give rise to projections that overlap extensively in the striatum. Anatomical studies by Ann Graybiel and her colleagues at Massachusetts Institute of Technology have shown that cortical regions concerned with the hand (see Chapter 9) converge in specific rostrocaudal bands within the striatum; conversely, regions in the same cortical areas concerned with the leg converge in other striatal bands. These rostrocaudal bands, therefore, appear to be functional units concerned with the movement of particular body parts. Another study by the same group showed that the more extensively cortical areas are interconnected, the greater the overlap in their projections to the striatum. Projections from the Basal Ganglia to Other Brain Regions The medium spiny neurons of the caudate and putamen give rise to inhibitory GABAergic projections that terminate in another pair of nuclei in the basal ganglia complex: the internal division of the globus pallidus and a specific region of the substantia nigra called pars reticulata (because, unlike the pars compacta, axons passing through give it a reticulated appearance). These nuclei are in turn the major sources of the output from the basal ganglia (Figure 18.5). The globus pallidus and substantia nigra pars reticulata have similar output functions. In fact, developmental studies show that pars reticulata is actually part of the globus pallidus, although the two eventually become separated by fibers of the internal capsule. The striatal projections to these two nuclei resemble the corticostriatal pathways in that they terminate in rostrocaudal bands, the locations of which vary with the locations of their source in the striatum. A striking feature of the projections from the medium spiny neurons to the globus pallidus and substantia nigra is the degree of their convergence onto pallidal and reticulata cells. In humans, for example, the corpus striatum contains approximately 100 million neurons, about 75% of which are medium spiny neurons. In contrast, the main target of these neurons, the globus pallidus, comprises only about 700,000 cells. Thus, on average, more than 100 medium spiny neurons innervate each pallidal cell. The efferent neurons of the internal globus pallidus and substantia nigra pars reticulata together give rise to the major pathways that link the basal ganglia with upper motor neurons located in the cortex and in the brainstem (see Figure 18.5). The pathway to the motor cortex arises primarily in the internal globus pallidus and is relayed via the ventral anterior and ventral lateral nuclei of the dorsal thalamus. These thalamic nuclei project directly to motor areas of the cortex, thus completing a vast loop that originates in multiple cortical areas and terminates, after relays in the basal ganglia and thalamus, back in the motor areas of the frontal lobe. In contrast, the axons from substantia nigra pars reticulata synapse on upper motor neurons in the superior colliculus that command eye movements, without any intervening relay in the thalamus (see Figure 18.5 and Chapter 20). This difference between the globus pallidus and substantia nigra pars reticulata is not absolute, however, since many reticulata axons also project to the thalamus where they contact relay neurons that project to the frontal eye fields of the premotor cortex. Because the efferent cells of both the globus pallidus and substantia nigra pars reticulata are GABAergic, the main output of the basal ganglia is inhibitory . In contrast to the quiescent medium spiny neurons, the neurons in both these output zones have high levels of spontaneous activity that tend to prevent unwanted movements by tonically inhibiting the superior colliculus and thalamus. Since the medium spiny neurons of the striatum also are GABAergic and inhibitory, the net effect of the excitatory inputs that reach the striatum from the cortex is to inhibit the tonically active inhibitory cells of the globus pallidus and substantia nigra pars reticulata (Figure 18.6). Thus, in the absence of body movements, the globus pallidus neurons, for example, provide tonic inhibition to the relay cells in the ventral lateral and anterior nuclei of the thalamus. When the pallidal cells are inhibited by activity of the medium spiny neurons, the thalamic neurons are disinhibited and can relay signals from other sources to the upper motor neurons in the cortex. This disinhibition is what normally allows the upper motor neurons to send commands to local circuit and lower motor neurons that initiate movements. Conversely, an abnormal reduction in the tonic inhibition as a consequence of basal ganglia dysfunction leads to excessive excitability of the upper motor neurons, and thus to the involuntary movement syndromes that are characteristic of basal ganglia disorders such as Huntington's disease (Box A; see also Figure 18.9B).
- Figure 43-5 The somatotopic organization of the basal ganglia-thalamocortical motor circuit is illustrated in these mesial and lateral views of a monkey brain, as well as the basal ganglia and thalamus. The motor circuit is divided into a “face” representation (blue), “arm” representation (dark green), and “leg” representation (light green). Arrows show subcircuits within the portion of the motor circuit concerned with the arm. CM = centromedian nucleus of the thalamus; GPe = external segment of the globus pallidus; GPi = internal segment of the globus pallidus; MC = primary motor cortex; PMC = prefrontal motor cortex; SMA = supplementary motor area; STN = subthalamic nucleus; VApc = parvocellular portion of the ventral anterior nucleus of the thalamus; VLo = pars oralis of the ventrolateral nucleus of the thalamus. The Skeletomotor Circuit Engages Specific Portions of the Cerebral Cortex, Basal Ganglia, and Thalamus Since movement disorders are prominent in diseases of the basal ganglia, it is appropriate here to focus on the skeletomotor circuit. In primates the skeletomotor circuit originates in the cerebral cortex in precentral motor fields and postcentral somatosensory areas and projects largely to the putamen. The putamen is thus an important site for integration of movement related and sensory feedback information related to movement. The putamen receives topographic projections from the primary motor cortex and premotor areas, including the arcuate premotor area and the supplementary motor area. Somatosensory areas 3a, 1, 2, and 5 project in an overlapping manner to the motor portions of the putamen. Topographically organized projections from each cortical area result in a somatotopic organization of movement-related neurons in the putamen. The leg is represented in a dorsolateral zone, the orofacial region in a ventromedial zone, and the arm in a zone between the two (Figure 43-5). Each of these representations extends along virtually the entire rostrocaudal axis of the putamen. Recent anatomical and physiological data indicate that the skeletomotor circuit is further subdivided into several independent subcircuits, each centered on a specific precentral motor field. Output neurons in the putamen project topographically to the caudoventral portions of both segments of the pallidum and to the caudolateral portions of the substantia nigra pars reticulata. In turn, the motor portions of the internal pallidal segment and substantia nigra pars reticulata send topographic projections to specific thalamic nuclei, including three ventral nuclei—the ventral lateral nucleus (pars oralis) and the lateral ventral anterior nuclei (pars parvocellularis and pars magnocellularis)—and the centromedian nucleus (see Figure 18-4 for the organization of the thalamic nuclei). The skeletomotor circuit is then closed by projections from the ventral lateral and ventral anterior (pars magnocellularis) nuclei to the supplementary motor area, from the lateral ventral anterior (pars parvocellularis) and the ventral lateral nuclei to the premotor cortex, and from the ventral lateral and centromedian nuclei to the precentral motor fields. Single Cell Recording Studies Provide Direct Insight into the Role of the Motor Circuits The contribution of the basal ganglia to movement can be assessed most directly by studying the activity of neurons within the skeletomotor circuit of behaving primates, especially activity in the internal segment of the pallidum, the principal output nucleus. The onset of rapid, stimulus-triggered limb movements is proceeded first by changes in neuronal firing in the motor circuits of the cortex and only later in the basal ganglia. This sequential firing suggests that a serial processing occurs within the basal ganglia-thalamocortical circuits and that much of the activity within these circuits is initiated at the cortical level. During the execution of a specific motor act, such as wrist flexion or extension, the normally high rate of spontaneous discharge in movement-related neurons in the internal pallidal segment becomes even higher in the majority of cells, but in some it decreases. Neurons that exhibit phasic decreases in discharge may play a crucial role in movement by disinhibiting the ventrolateral thalamus and thereby gating or facilitating cortically initiated movements (via excitatory thalamocortical connections). Populations of neurons that show phasic increases in discharge would have the opposite effect, further inhibiting thalamocortical neurons and thus suppressing antagonistic or competing movements. Little is known about how movement-related signals from the direct and indirect pathways are integrated in the internal pallidal segment to control basal ganglia output. One possibility, of course, is that signals associated with a particular voluntary movement are directed over both pathways to the same population of pallidal neurons. With this arrangement, the inputs from the indirect pathway might assist in braking or possibly smoothing the movement, while those in the direct pathway simultaneously facilitate the movement. This reciprocal regulation would be consistent with the basal ganglia's apparent role in scaling the amplitude or velocity of movement. Alternatively, the direct and indirect inputs associated with a particular movement could be directed to separate sets of neurons in the output nuclei of the basal ganglia. In this configuration, the skeletomotor circuit might play a dual role in modulating voluntary movements by both reinforcing the selected pattern (via the direct pathway) and suppressing potentially conflicting patterns (via the indirect pathway). This dual role could result in focusing the neural activity that mediates each voluntary movement in a way similar to the inhibitory surround described for various sensory systems. Neuronal activity within the skeletemotor circuit has been examined in monkeys performing a variety of motor tasks. At all stages of the circuit (cortical, striatal, and pallidal) the activity of substantial proportions of movement-related neurons depends upon the direction of limb movement, independent of the pattern of muscle P.860 P.861 activity. These directional cells comprise 30-50% of the movement-related neurons in the supplementary motor area, motor cortex, putamen, and pallidum. All of these neurons are arranged somatotopically. In the motor cortical, but not in the basal ganglia many movement-related cells have been found whose firing does depend on the pattern of muscle activity. In trained primates, the activity in arm-related neurons of the internal pallidal segment also is clearly correlated with amplitude and velocity. Studies combining behavioral training and single-cell recording indicate that the skeletomotor circuit is involved not only in the execution but also in the prepartion for movement. In the precentral motor fields, including the premotor cortex, supplementary motor area, and motor cortex, striking changes in discharge rate occur in some neurons after the presentation of a cue that specifies the direction of limb movement to be executed later. These changes in activity persist until the movement-triggering stimulus is presented. They thus represent a neural correlate of one of the preparatory aspects of motor control referred to as “motor set” (Chapter 38). Directionally selective activity before movement also occurs within the putamen and the internal segment of the pallidum. Individual neurons within these structures tend to exhibit either preparatory (set-related) or movement-related responses, suggesting that the preparation and execution of motor action are mediated by separate subchannels in the skeletomotor circuit. In the internal segment of the pallidum subpopulations of neurons that receive input from the supplementary motor area tend to exhibit set-like preparatory responses. However, neurons receiving inputs from the motor cortex tend to exhibit phasic, movement-related responses. These different response patterns further support the idea that the skeletomotor circuit is composed of distinct subcircuits that connect to different precentral motor fields (motor cortex, supplementary motor area, and arcuate premotor area). These subcircuits may have distinctive roles in motor control and in the pathogenesis of specific motor signs and symptoms that occur in Parkinson disease and other diseases of the basal ganglia.
- Figure 18.6. A chain of nerve cells arranged in a disinhibitory circuit. Top: Diagram of the connections between two inhibitory neurons, A and B, and an excitatory neuron, C. Bottom: Pattern of the action potential activity of cells A, B, and C when A is at rest, and when neuron A fires transiently as a result of its excitatory inputs. Such circuits are central to the gating operations of the basal ganglia.
- Figure 18.8. Disinhibition in the direct and indirect pathways through the basal ganglia. (A) In the direct pathway, transiently inhibitory projections from the caudate and putamen project to tonically active inhibitory neurons in the internal segment of the globus pallidus, which project in turn to the VA/VL complex of the thalamus. Transiently excitatory inputs to the caudate and putamen from the cortex and substantia nigra are also shown, as is the transiently excitatory input from the thalamus back to the cortex. (B) In the indirect pathway (shaded by yellow), transiently active inhibitory neurons from the caudate and putamen project to tonically active inhibitory neurons of the external segment of the globus pallidus. Note that the influence of nigral dopaminergic input to neurons in the indirect pathway is inhibitory. The globus pallidus (external segment) neurons project to the subthalamic nucleus, which also receives a strong excitatory input from the cortex. The subthalamic nucleus in turn projects to the globus pallidus (internal segment), where its transiently excitatory drive acts to oppose the disinhibitory action of the direct pathway. In this way, the indirect pathway modulates the effects of the direct pathway. A further indication of functional subdivision within the striatum is the spatial distribution of different types of medium spiny neurons. Although medium spiny neurons are distributed throughout the striatum, they occur in clusters of cells called "patches" or "striosomes" and in a surrounding "matrix" of neurochemically distinct cells. Whereas the distinction between the patches and matrix was originally based only on differences in the types of neuropeptides contained by the medium spiny cells in the two regions, the cell types are now known to differ in the sources of their inputs from the cortex and in the destinations of their projections to other parts of the basal ganglia. For example, even though most cortical areas project to medium spiny neurons in both these compartments, limbic areas of the cortex (such as the cingulate gyrus) project more heavily to the patches, whereas motor and somatic sensory areas project preferentially to the neurons in the matrix. These differences in the connectivity of medium spiny neurons in the patches and matrix further support the conclusion that functionally distinct pathways project in parallel from the cortex to the striatum. The nature of the signals transmitted to the caudate and putamen from the cortex is not understood. It is known, however, that collateral axons of the corticocortical, corticothalamic, and corticospinal pathways all make excitatory glutamatergic synapses on the dendritic spines of medium spiny neurons (see Figure 18.3B ). The arrangement of these cortical synapses is such that the number of contacts established between an individual cortical axon and a single medium spiny cell is very small, whereas the number of spiny neurons contacted by a single axon is extremely large. This divergence of cortical axon terminals allows a single medium spiny neuron to integrate the influences of thousands of cortical cells. The medium spiny cells also receive noncortical inputs from interneurons, from the midline and intralaminar nuclei of the thalamus, and from most of the brainstem aminergic nuclei. In contrast to the cortical inputs, the motor circuit neuron and thalamic synapses are made on the dendritic shafts and close to the cell soma, where they can modulate the effectiveness of cortical synaptic activation arriving from the more distal dendrites. The aminergic ones are dopaminergic synapses from a subdivision of the substantia nigra called pars compacta because of its densely packed cells. These synapses are, in contrast, located on the base of the spines in close proximity to the cortical synapses, where they more directly modulate cortical input (see Figure 18.3B ). As a result, inputs from both the cortex and the substantia nigra pars compacta are relatively far from the initial segment of the medium spiny neuron axon, where the nerve impulse is generated. Accordingly, the medium spiny neurons must simultaneously receive many excitatory inputs from cortical and nigral neurons in order to become active. The medium spiny neurons are, therefore, usually silent. When the medium spiny neurons do become active, their activity is associated with the imminent occurrence of a movement. Extracellular recordings show that these neurons typically increase their rate of discharge just before an impending movement. Neurons in the putamen tend to discharge in anticipation of body movements, whereas caudate neurons fire prior to eye movements. These anticipatory discharges are evidently part of a movement selection process; in fact, they can precede the initiation of movement by as much as several seconds. Similar recordings have also shown that the discharges of some striatal neurons vary according to the location in space of the target of a movement, rather than with the starting position of the limb relative to the target. Thus, the activity of these cells may encode the decision to reach toward the target, rather than simply the direction and amplitude of a movement as such. Circuits within the Basal Ganglia System The projections from the medium spiny neurons of the caudate and putamen to the internal segment of the globus pallidus and substantia nigra pars reticulata are part of a "direct pathway" and, as just described, serve to release the upper motor neurons from tonic inhibition. This pathway is summarized in Figure 18.8A. A second pathway serves to increase the level of tonic inhibition and is called the "indirect pathway" (Figure 18.8B). This pathway provides a second route linking the corpus striatum with the internal globus pallidus and substantia nigra pars reticulata. In the indirect pathway, another population of medium spiny neurons projects to the lateral or external segment of the globus pallidus . This external division sends projections to both the internal segment of the globus pallidus and the subthalamic nucleus of the ventral thalamus (see Figure 18.1). But, instead of projecting to structures outside of the basal ganglia, the subthalamic nucleus projects back to the internal segment of the globus pallidus and to the substantia nigra pars reticulata. As already described, these latter two nuclei project out of the basal ganglia, which thus allows the indirect pathway to influence the activity of the upper motor neurons. The indirect pathway through the basal ganglia apparently serves to modulate the disinhibitory actions of the direct pathway. The subthalamic nucleus neurons that project to the internal globus pallidus and substantia nigra pars reticulata are excitatory. Normally, when the indirect pathway is activated by signals from the cortex, the medium spiny neurons discharge and inhibit the tonically active GABAergic neurons of the external globus pallidus. As a result, the subthalamic cells become more active and, by virtue of their excitatory synapses with cells of the internal globus pallidus and reticulata, they increase the inhibitory outflow of the basal ganglia. Thus, in contrast to the direct pathway, which when activated releases tonic inhibition, the net effect of activity in the indirect pathway is to increase inhibitory influences on the upper motor neurons. The indirect pathway can thus be regarded as a "brake" on the normal function of the direct pathway. Indeed, many neural systems achieve fine control of their output by a similar interplay between excitation and inhibition. The consequences of imbalances in this fine control mechanism are apparent in diseases that affect the subthalamic nucleus. These disorders remove a source of excitatory input to the internal globus pallidus and reticulata, and thus abnormally reduce the inhibitory outflow of the basal ganglia. A basal ganglia syndrome called hemiballismus , which is characterized by violent, involuntary movements of the limbs, is the result of damage to the subthalamic nucleus. The involuntary movements are initiated by abnormal discharges of upper motor neurons that are receiving less tonic inhibition from the basal ganglia. Another circuit within the basal ganglia system entails the dopaminergic cells in the pars compacta subdivision of substantia nigra and modulates the output of the corpus striatum. The medium spiny neurons of the corpus striatum project directly to substantia nigra pars compacta, which in turn sends widespread dopaminergic projections back to the spiny neurons. These dopaminergic influences on the spiny neurons are complex: The same nigral neurons can provide excitatory inputs mediated by D1 type dopaminergic receptors on the spiny cells that project to the internal globus pallidus (the direct pathway), and inhibitory inputs mediated by D2 type receptors on the spiny cells that project to the external globus pallidus (the indirect pathway). Since the actions of the direct and indirect pathways on the output of the basal ganglia are antagonistic, these different influences of the nigrostriatal axons produce the same effect, namely a decrease in the inhibitory outflow of the basal ganglia. The modulatory influences of this second internal circuit help explain many of the manifestations of basal ganglia disorders. For example, Parkinson's Disease is caused by the loss of the nigrostriatal dopaminergic neurons (Figure 18.9A and Box B). As mentioned earlier, the normal effects of the compacta input to the striatum are excitation of the medium spiny neurons that project directly to the internal globus pallidus and inhibition of the spiny neurons that project to the external globus pallidus cells in the indirect pathway. Normally, both of these dopaminergic effects serve to decrease the inhibitory outflow of the basal ganglia and thus to increase the excitability of the upper motor neurons (Figure 18.10A). In contrast, when the compacta cells are destroyed, as occurs in Parkinson's disease, the inhibitory outflow of the basal ganglia is abnormally high, and thalamic activation of upper motor neurons in the motor cortex is therefore less likely to occur. In fact, many of the symptoms seen in Parkinson's disease (and in other hypokinetic movement disorders) reflect a failure of the disinhibition normally mediated by the basal ganglia. Thus, Parkinsonian patients tend to have diminished facial expressions and lack "associated movements" such as arm swinging during walking. Indeed, any movement is difficult to initiate and, once initiated, is often difficult to terminate. Disruption of the same circuits also increases the discharge rate of the inhibitory cells in substantia nigra pars reticulata. The resulting increase in tonic inhibition reduces the excitability of the upper motor neurons in the superior colliculus and causes saccades to be reduced in both frequency and amplitude. Support for this explanation of hypokinetic movement disorders like Parkinson's disease comes from studies of monkeys in which degeneration of the dopaminergic cells of substantia nigra has been induced by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Monkeys (or humans) exposed to MPTP develop symptoms that are very similar to those of patients with Parkinson's disease. Furthermore, a second lesion placed in the subthalamic nucleus results in significant improvement in the ability of these animals to initiate movements, as would be expected based on the circuitry of the indirect pathway (see Figure 18.8B). Similarly, knowledge about the indirect pathway within the basal ganglia helps explain the motor abnormalities seen in Huntington's disease (see Box A). In patients with Huntington's disease, medium spiny neurons that project to the external segment of the globus pallidus degenerate (see Figure 18.9B). In the absence of their normal inhibitory input from the spiny neurons, the external globus pallidus cells become abnormally active; this activity reduces in turn the excitatory output of the subthalamic nucleus to the internal globus pallidus (Figure 18.10B). In consequence, the inhibitory outflow of the basal ganglia is reduced. Without the restraining influence of the basal ganglia, upper motor neurons can be activated by inappropriate signals, resulting in the undesired ballistic and choreic (dancelike) movements that characterize Huntington's disease. Importantly, the basal ganglia may exert a similar influence on other non-motor systems with equally significant clinical implications (Box C). As predicted by this account, GABA agonists and antagonists applied to substantia nigra pars reticulata of monkeys produce symptoms similar to those seen in human basal ganglia disease. For example, intranigral injection of bicuculline, which blocks the GABAergic inputs from the striatal medium spiny neurons to the reticulata cells, increases the amount of tonic inhibition on the upper motor neurons in the deep collicular layers. These animals exhibit fewer, slower saccades, reminiscent of patients with Parkinson's disease. In contrast, injections of the GABA agonist muscimol into substantia nigra pars reticulata decrease the tonic GABAergic inhibition of the upper motor neurons in the superior colliculus, with the result that the injected monkeys generate spontaneous, irrepressible saccades that resemble the involuntary movements characteristic of basal ganglia diseases such as hemiballismus and Huntington's disease (Figure 18.11). Summary The contribution of the basal ganglia to motor control is apparent from the deficits that result from damage to the component nuclei. Such lesions compromise the initiation and performance of voluntary movements, as exemplified by the paucity of movement in Parkinson's disease and in the inappropriate "release" of movements in Huntington's disease. The organization of the basic circuitry of the basal ganglia indicates how this constellation of nuclei modulates movement. With respect to motor function, the system forms a loop that originates in almost every area of the cerebral cortex and eventually terminates, after enormous convergence within the basal ganglia, on the upper motor neurons in the motor and premotor areas of the frontal lobe and the superior colliculus. The efferent neurons of the basal ganglia influence the upper motor neurons in the cortex by gating the flow of information through relays in the ventral nuclei of the thalamus. The upper motor neurons in the superior colliculus that initiate saccadic eye movements are controlled by monosynaptic projections from substantia nigra pars reticulata. In each case, the basal ganglia loop regulates movement by a process of disinhibition that results from the serial interaction within the basal ganglia circuitry of two GABAergic neurons. Internal circuits within the basal ganglia system modulate the amplification of the signals that are transmitted through the loop. The Basal Ganglia Also Have a Role in Cognition, Mood, and Nonmotor Behavior Function Some circuits in the basal ganglia are involved in nonmotor aspects of behavior. These circuits originate in the prefrontal and limbic regions of the cortex and engage specific areas of the striatum, pallidum, and substantia nigra. The dorsolateral prefrontal circuit originates in Brodmann's areas 9 and 10 and projects to the head of the caudate nucleus, which then projects directly and indirectly to the dorsomedial portion of the internal pallidal segment and the rostral substantia nigra pars reticulata. Projections from these regions terminate in the ventral anterior and medial dorsal thalamic nuclei, which in turn project back upon the dorsolateral prefrontal area. The dorsolateral prefrontal circuit has been implicated broadly in so-called “executive functions” (Chapter 19). These include cognitive tasks such as organizing behavioral responses and using verbal skills in problem solving. Damage to the dorsolateral prefrontal cortex or subcortical portions of the circuit is associated with a variety of behavioral abnormalities related to these cognitive functions. The lateral orbitofrontal circuit arises in the lateral prefrontal cortex and projects to the ventromedial caudate nucleus. The pathway from the caudate nucleus follows that of the dorsolateral circuit (through the internal pallidal segment and substantia nigra pars reticulata and thence to the thalamus) and returns to the orbitofrontal cortex. The lateral orbitofrontal cortex appears to play a major role in mediating empathetic and socially appropriate responses. Damage to this area is associated with irritability, emotional lability, failure to respond to social cues, and lack of empathy. A neuro-psychiatric disorder thought to be associated with disturbances in the lateral orbitofrontal cortex and circuit is obsessive-compulsive disorder (Chapter 61). The anterior cingulate circuit arises in the anterior cingulate gyrus and projects to the ventral striatum. The ventral striatum also receives “limbic” input from the hippocampus, amygdala, and entorhinal cortices. The projections of the ventral striatum are directed to the ventral and rostromedial pallidum and the rostrodorsal substantia nigra pars reticulata. From there the pathway continues to neurons in the paramedian portion of the medial dorsal nucleus of the thalamus, which in turn project back upon the anterior cingulate cortex. The anterior cingulate circuit appears to play an important role in motivated behavior, and it may convey reinforcing stimuli to diffuse areas of the basal ganglia and cortex via inputs through the ventral tegmental areas and the substantia nigra pars compacta. These inputs may play a major role in procedural learning (see Chapter 62). Damage to the anterior cingulate region bilaterally can cause akinetic mutism, a condition characterized by profound impairment of movement initiation. In general, the disorders associated with dysfunction of the prefrontal cortex and corticobasal ganglia-thalamocortical circuits involve action rather than of perception or sensation. These disturbances are associated both with either intensified action (impulsivity) and flattened action (apathy). Obsessive-compulsive behavior can be viewed as a form of hyperactivity. The disturbances of mood associated with circuit dysfunction are believed to span the extremes of mania and depression. Both dopamine and serotonin, two biogenic amines that modulate neuronal activity within the circuits, are important to depression (Chapter 61). These observations suggest that the neural mechanisms underlying complex behavioral disorders might be analogous to the dysfunctions of the motor circuits described in this chapter. Thus, schizophrenia might be viewed as a “Parkinson disease of thought.” By this analogy, schizophrenic symptoms would arise from disordered modulation of prefrontal circuits. Other cognitive and emotional symptoms may similarly be equivalents of motor disturbances such as tremor, dyskinesia, and rigidity. An Overall View In 1949 Linus Pauling revolutionized medical thinking by coining the term “molecular disease.” He and his collaborators observed the altered electrophoretic mobility of hemoglobin S and reasoned that sickle cell anemia, a disease known to be genetic, could be explained by a mutation of a gene for a specific protein. A decade later Vernon Ingram showed that this alteration in charge occurs in the amino acid sequence of hemoglobin S, where a glutamic acid residue is replaced by a valine. This change from a single negatively charged residue in normal hemoglobin to a neutral one explains the altered molecular properties of hemoglobin S, and these in turn account for the intermolecular differences and disordered cell stacking observed in sickled red cells. Thus, a single molecular change is fundamental to understanding the patient's pathology, symptoms, and prognosis. While the explanation for other diseases may not be as simple, it is a fundamental principle of modern medicine that every disorder has a molecular basis. Research in Parkinson disease and myasthenia gravis first made the medical community realize that particular components of chemical synapses can be specific targets for disease. In myasthenia gravis the molecular target is the acetylcholine receptor. In the disorders of the basal ganglia some components of the synthesis, packaging, or turnover of dopamine and serotonin are altered. The causes of the pathological alterations of these loci, whether genetic, infectious, toxic, or degenerative, are not yet known. Although we have identified the mutant gene for Huntington disease, as yet we have no idea about the function of the protein that the wild-type gene encodes. It is clear that rational treatment for diseases of transmitter metabolism requires a good understanding of synaptic transmission in the affected pathways.
- Figure 19.2. Components of the brainstem and diencephalon related to the cerebellum. This sagittal section shows the major structures of the cerebellar system, including the cerebellar cortex, the deep cerebellar nuclei, and the ventroanterior and ventrolateral (VA/VL) complex (which is the target of some of the deep cerebellar nuclei). The Cerebellum Claude Ghez ,W. Thomas Thach THE CEREBELLUM (Latin, little brain) constitutes only 10% of the total volume of the brain but contains more than half of all its neurons. These neurons are arranged in a highly regular manner as repeating units, each of which is a basic circuit module. Despite its structural regularity the cerebellum is divided into several distinct regions, each of which receives projections from different portions of the brain and spinal cord and projects to different motor systems. These features suggest that regions of the cerebellum perform similar computational operations but on different inputs. The cerebellum influences the motor systems by evaluating disparities between intention and action and by adjusting the operation of motor centers in the cortex and brain stem while a movement is in progress as well as during repetitions of the same movement. Three aspects of the cerebellum's organization underlie this function. First, the cerebellum is provided with extensive information about the goals, commands, and feedback signals associated with the programming and execution of movement. The importance of this input is evident in the fact that 40 times more axons project into the cerebellum than exit from it. Second, the output projections of the cerebellum are focused mainly on the premotor and motor systems of the cerebral cortex and brain stem, systems that control spinal interneurons and motor neurons directly. Third, synaptic transmission in the circuit modules can be modified, a feature that is crucial for motor adaptation and learning. Organization of the Cerebellum The cerebellum can be subdivided into three main parts based on differences in the sources of input ( Figure 19.1 and Table 19.1 ). By far, the largest subdivision in humans is the cerebrocerebellum . It occupies most of the lateral cerebellar hemisphere and receives input from many areas of the cerebral cortex. This region of the cerebellum is especially well developed in primates. The cerebrocerebellum is concerned with the regulation of highly skilled movements, especially the planning and execution of complex spatial and temporal sequences of movement (including speech). The phylogenetically oldest part of the cerebellum is the vestibulocerebellum . This portion of the cerebellum comprises the caudal lobes of the cerebellum and includes the flocculus and the nodulus. As its name suggests, the vestibulocerebellum receives input from the vestibular nuclei in the brainstem and is primarily concerned with the regulation of movements underlying posture and equilibrium. The last of the major subdivisions is the spinocerebellum . The spinocerebellum occupies the median and paramedian zone of the cerebellar hemispheres and is the only part that receives input directly from the spinal cord. The lateral part of the spinocerebellum is primarily concerned with movements of distal muscles, such as the relatively gross movements of the limbs in walking. The central part, called the vermis , is primarily concerned with movements of proximal muscles, and also regulates eye movements in response to vestibular inputs. The connections between the cerebellum and other parts of the nervous system occur by way of three large pathways called cerebellar peduncles ( Figures 19.1 to 19.3 ). The superior cerebellar peduncle (or brachium conjunctivum) is almost entirely an efferent pathway. The neurons that give rise to this pathway are in the deep cerebellar nuclei, and their axons project to upper motor neurons in the red nucleus, the deep layers of the superior colliculus, and, after a relay in the dorsal thalamus, the primary motor and premotor areas of the cortex (see Chapter 17 ). The middle cerebellar peduncle (or brachium pontis) is an afferent pathway to the cerebellum; most of the cell bodies that give rise to this pathway are in the base of the pons, where they form the pontine nuclei ( Figure 19.2 ). The pontine nuclei receive input from a wide variety of sources, including almost all areas of the cerebral cortex and the superior colliculus. The axons of the pontine nuclei, called transverse pontine fibers , cross the midline and enter the cerebellum via the middle cerebellar peduncle ( Figure 19.3 ). Each of the two middle cerebellar peduncles contains over 20 million axons and are thus among the largest pathways in the brain. In comparison, the optic and pyramidal tracts contain only about a million axons. Most of these pontine axons relay information from the cortex to thecerebellum. Finally, the inferior cerebellar peduncle (or restiform body) is the smallest but most complex of the cerebellar peduncles, containing multiple afferent and efferent pathways. Efferent pathways in this peduncle project to the vestibular nuclei and the reticular formation; the afferent pathways include axons from the vestibular nuclei, the spinal cord, and several regions of the brainstem tegmentum The Cerebellum Has Three Functionally Distinct Regions The cerebellum occupies most of the posterior cranial fossa. It is composed of an outer mantle of gray matter (the cerebellar cortex ), internal white matter, and three Figure 42-2 The cerebellum is divided into anatomically distinct lobes. A. The cerebellum is unfolded to reveal the lobes normally hidden from view. B. The main body of cerebellum has three functional regions: the central vermis and the lateral and intermediate zones in each hemisphere. It is divided by the primary fissure into anterior and posterior lobes. The posterolateral fissure separates the flocculonodular lobe. Shallower fissures divide the anterior and posterior lobes into nine lobules (anatomists consider the flocculonodular lobe as the tenth lobule). pairs of deep nuclei: the fastigial , the interposed (itself composed of two nuclei, the globose and emboliform ), and the dentate (Figure 42-1). The cerebellum is connected to the dorsal aspect of the brain stem by three symmetrical pairs of tracts: the inferior cerebellar peduncle (also called the restiform body), the middle cerebellar peduncle (or brachium pontis), and the superior cerebellar peduncle (or brachium conjunctivum). The superior cerebellar peduncle contains most of the efferent projections. With one exception, cerebellar output originates from cell bodies in the deep nuclei. The exception is a relatively small portion of cerebellar cortex—the flocculonodular lobe —whose cells project to the lateral and medial vestibular nuclei in the brain stem. A striking feature of the surface of the cerebellum is the presence of many parallel convolutions called folia (Latin, leaves) that run from side to side (Figure 42-1). Two deep transverse fissures divide the cerebellum into three lobes. The primary fissure on the dorsal surface separates the anterior and posterior lobes, which together form the body of the cerebellum. The posterolateral fissure on the ventral surface separates the body from the much smaller flocculonodular lobe (Figure 42-2). Sagittal section through the midline shows that shallower fissures further subdivide each lobe into several lobules comprising a variable number of folia. Two longitudinal furrows, which are most prominent ventrally, distinguish three mediolateral regions that are important functionally. The furrows define an elevated ridge in the midline known as the vermis (Latin, worm). On either side of the vermis are the cerebellar hemispheres , each of which is divided into intermediate and lateral regions (Figure 42-2). The three mediolateral regions of the body of the cerebellum (the vermis and intermediate and lateral parts of the hemispheres) and the flocculonodular lobe receive different afferent inputs, project to different parts of the motor systems, and represent distinct functional subdivisions. The flocculonodular lobe is the most primitive part of the cerebellum, appearing first in fishes. Its cortex receives input directly from primary vestibular afferents and projects to the lateral vestibular nuclei (Figure 42-3). In higher vertebrates its function is limited to controlling balance and eye movements and is thus called the vestibulocerebellum. The vermis and hemispheres develop later in phylogeny. The vermis receives visual, auditory, and vestibular input as well as somatic sensory input from the head and proximal parts of the body. It projects by way of the fastigial nucleus to cortical and brain stem regions that give rise to the medial descending systems that control proximal muscles of the body and limbs. The vermis governs posture and locomotion as well as gaze. The adjacent intermediate part of the hemisphere also receives somatosensory input from the limbs. This region projects via the interposed nucleus to lateral corticospinal and rubrospinal systems and thus controls the more distal muscles of the limbs and digits. Because the vermis and intermediate hemispheres are the only regions to receive somatosensory inputs from the spinal cord, they are often called the spinocerebellum. The lateral parts of the hemispheres, which are phylogenetically most recent, are much larger in humans and apes than in monkeys or cats. This region receives input exclusively from the cerebral cortex and is thus called the cerebrocerebellum. Its output is mediated by the dentate nucleus, which projects to motor, premotor, and prefrontal cortices. Recent imaging data indicate that the cerebrocerebellum is intimately involved in planning and mental rehearsal of complex motor actions and in the conscious assessment of movement errors.
- Figure 42-3 The three functional regions of the cerebellum have different inputs and outputs. Figure 19.3. Functional organization of the inputs to the cerebellum. (A) Diagram of the major inputs. (B) Idealized coronal and sagittal sections through the human brainstem and cerebrum, showing inputs to the cerebellum from the cortex, vestibular system, spinal cord, and brainstem. The cortical projections to the cerebellum are made via relay neurons in the pons. These axons then cross the midline within the pons and run to the cerebellum via the middle cerebellar peduncle. Axons from the inferior olive, spinal cord, and vestibular nuclei enter via the inferior cerebellar peduncle.
- Figure 19.6. Functional organization of the outputs from the cerebellum to the cerebral cortex. (A) Diagram of this aspect of the targets of the cerebellum. The axons of the deep cerebellar nuclei cross in the midbrain in the decussation of the superior cerebellar peduncle before reaching the thalamus. (B) Idealized coronal and sagittal sections through the human brainstem and cerebrum, showing the location of the structures and pathways diagrammed in (A). Projections from the Cerebellum Except for a direct projection from the vestibulocerebellum to the vestibular nuclei, the cerebellar cortex projects to the deep cerebellar nuclei, which project in turn to upper motor neurons in the cortex (via a relay in the thalamus) and spinal cord (via relays in the brainstem) ( Figure 19.6 and Table 19.3 ). There are four major deep nuclei: the dentate nucleus (by far the largest), two interposed nuclei, and the fastigial nucleus . Each receives input from a different region of the cerebellar cortex. Although the borders are not distinct, in general, the cerebrocerebellum projects primarily to the dentate nucleus, the spinocerebellum to the interposed nuclei, and the vestibulocerebellum to the fastigial nucleus. Axons from the dentate nucleus are destined for the cortex via a projection to the ventral nuclear complex in the thalamus. This pathway must cross the midline if the motor cortex in each hemisphere, which is concerned with contralateral musculature, is to receive information from the cerebellum about the appropriate side of the body. Consequently, the dentate axons exit thecerebellum via the superior cerebellar peduncle, cross at the decussation of the superior cerebellar peduncle in the caudal midbrain, and then ascend to the thalamus. The thalamic nuclei that receive projections from the deep cerebellar nuclei are segregated in two distinct subdivisions of the ventral lateral nuclear complex: the oral, or anterior, part of the posterolateral segment, and a region simply called area X. Both of these thalamic relays project directly to primary motor and premotor association cortices. Thus, the cerebellum has access to the upper motor neurons that organize the sequence of muscular contractions underlying complex voluntary movements. Pathways leaving the deep cerebellar nuclei also project to upper motor neurons in the red nucleus, the superior colliculus, the vestibular nuclei, and the reticular formation (see Table 19.3 and Chapter 17 ). Anatomical studies using viruses to trace chains of connections between nerve cells have shown that large parts of the cerebrocerebellum send information back to non-motor areas of the cortex to form “closed loops.” That is, a region of the cerebellum projects back to the same cortical area that in turn projects to it. These closed loops run in parallel to “open loops” that receive input from multiple cortical areas and funnel output back to upper motor neurons in specific regions of the motor and premotor cortices ( Figure 19.7 )
- Figure 42-10 The vestibulocerebellum (flocculonodular lobe) receives input from the vestibular labyrinth and projects directly to the vestibular nuclei. (Oculomotor connections of the vestibular nuclei are omitted for clarity.) The vermis receives input from the neck and trunk, the vestibular labyrinth, and the retinal and extraocular muscles. Its output is focused on the ventromedial descending systems of both the brain stem (mainly the reticulospinal and vestibulospinal tracts) and cortex (corticospinal fibers acting on medial motor neurons). In contrast, climbing fibers fire spontaneously at very low rates, and these spontaneous rates are changed only modestly by sensory stimuli or during active movement. The frequency of complex spikes in Purkinje cells is rarely more than 1-3 per second. Such low frequencies cannot by themselves carry substantial information about the magnitude of natural stimuli or behavior. What could be encoded by the complex spikes? One possibility is that complex spikes might signal the timing of peripheral events or act as triggers for behavior. Rodolfo Llinás has suggested that a form of timing signal might be provided by the synchronous firing of multiple Purkinje cells. Neurons in the inferior olivary nucleus are often electrotonically connected to one another through dendrodendritic synapses and therefore can fire in synchrony. The synchronous inputs of olivary neurons in climbing fibers produces complex spikes in many Purkinje cells at almost the same time. Interestingly, the electrotonic coupling of olivary neurons is under efferent control by GABA-ergic fibers from the cerebellar nuclei terminating in the olivary nucleus (Figure 42-6). By functionally disconnecting certain olivary neurons through inhibition the nervous system could be selecting a specific array of Purkinje neurons for synchronous activation. This idea is supported by cell recordings in which different patterns of synchronous discharge in different sets of Purkinje neurons are correlated with different phases of a natural behavior (Figure 42-9). Thus, although there is little divergence of climbing fibers, synchronization of inputs may allow populations of postsynaptic neurons with different inputs to act cooperatively. Climbing Fiber Activity Produces Long-Lasting Effects on the Synaptic Efficacy of Parallel Fibers Despite the low frequency of their discharge, climbing fibers may alter cerebellar output by modulating the synaptic effect of parallel fiber input to Purkinje cells in two ways. First, climbing fiber action potentials slightly reduce the strength of the parallel fiber input to the Purkinje cell. Thus, experimental lesions or localized cooling of the inferior olivary nucleus produce a large increase in the frequency of simple spikes generated in the Purkinje cells by the parallel fibers. Second, activity in climbing fibers can induce selective long-term depression in the synaptic strength of parallel fibers that are active concurrently. Long-term depression has been analyzed in slices of cerebellum in which Purkinje cell responses to concurrent stimulation of climbing fibers and parallel fibers can be recorded intracellularly. Masao Ito and co-workers found that concurrent stimulation of climbing fibers and one set of parallel fibers depresses the effect of later stimulation of the P.841 same parallel fibers but has no effect on the stimulation of another set of parallel fibers. For this depression to occur, however, the parallel fiber's simple spike must occur within some 100-200 ms of the climbing fiber's complex spike. The resulting depression can last minutes to hours and depends critically on the prolonged depolarization and large influx of calcium produced by the climbing fiber in Purkinje cell dendrites. This long-term effect of the climbing fiber on the transmission of signals from the mossy fiber, granule cell, and parallel fiber through to the Purkinje cell may be important in the cerebellar role in motor learning. The Vestibulocerebellum Regulates Balance and Eye Movements The vestibulocerebellum (flocculonodular lobe) receives information from the semicircular canals and the otolith organs, which sense motion of the head and its position relative to gravity (Chapter 40). Mossy fibers that terminate in the vestibulocerebellar cortex arise from neurons in the vestibular nuclei. The vestibulocerebellar cortex also receives visual input via mossy fibers from the superior colliculi and from the striate cortex, the latter relayed through the pontine nuclei. Purkinje neurons in the vestibulocerebellum inhibit neurons in the medial and lateral vestibular nuclei. Through the lateral nucleus they modulate the lateral and medial vestibulospinal tracts, which predominantly control axial muscles and limb extensors, assuring balance during stance and gait (Figure 42-10). The inhibitory projection to the medial vestibular nucleus controls eye movements and coordinates movements of the head and eyes via the medial longitudinal fasciculus (Chapter 41). Disruption of these projections through lesions or disease impairs an individual's ability to use vestibular information to control eye movements during head rotations and movements of the limbs and body during standing and walking. Patients have difficulty maintaining balance; they attempt to compensate by separating their feet widely while standing or walking, thus increasing their base of support. They move their legs irregularly and often fall, whether their eyes are open or closed. In contrast, patients have no difficulty moving their arms or legs accurately while lying down or when their body and head are supported. This test indicates that the primary difficulty is in using vestibular cues for standing and walking, not in controlling the limbs for all movements.
- Figure 42-12 Neurons in the intermediate and lateral parts of the cerebellar hemisphere project to the contralateral red nucleus and motor cortex. The intermediate zone (spinocerebellum) receives sensory information from the limbs and controls the dorsolateral descending systems (rubrospinal and corticospinal tracts) acting on the ipsilateral limbs. There is a modest projection from the fastigial nucleus to the ventrolateral nucleus in the thalamus and the primary motor cortex. The lateral zone (cerebrocerebellum) receives cortical input via the pontine nuclei and influences the motor and premotor cortices via the ventrolateral nucleus of the thalamus. Direct pathways originate from interneurons in the spinal gray matter and terminate as mossy fibers in the vermis or intermediate cortex. Two important pathways are the ventral and dorsal spinocerebellar tracts. These pathways from spinal interneurons provide the cerebellum with somatic sensory information from the legs— notably from muscle and joint proprioceptors—and with information about descending commands reaching the interneurons. Recordings from neurons in the dorsal and ventral spinocerebellar tracts of decerebrate cats walking on a treadmill show that both systems are modulated rhythmically and in phase with the step cycle. However, when the dorsal roots are cut, preventing spinal neurons from receiving phase-dependent peripheral excitation, dorsal spinocerebellar neurons fall silent while ventral spinocerebellar neurons continue to be modulated. This finding demonstrates that the ventral tract carries internally generated information about the central locomotor rhythm as well as the rhythmic discharge of somatic sensory receptors, while the dorsal tract provides the cerebellum with sensory feedback only during evolving movements. Other direct pathways provide comparable information from the upper extremities. Direct pathways from the spinal cord to the cerebellum synapse first with neurons in one of several so-called precerebellar nuclei in the brain stem reticular formation (the lateral reticular nucleus, reticularis tegmenti pontis, and paramedian reticular nucleus). These inputs provide the cerebellum with different versions of the changing state of the organism and its environment and permit comparisons between such signals. Similar monitoring of outgoing commands is as crucial for perception as for movement, since the internal sensory signals resulting from movement must be distinguished from the external sensory signals in the environment. The Spinocerebellum Contains Sensory Maps The initial mapping studies of the spinocerebellum by Edgar Adrian and Ray Snider in the 1940s revealed two inverted somatotopic maps. In both maps the head is represented in the posterior vermis, and the repre-sentations of the neck and trunk extend on either side along the dorsal and ventral portions of the vermis. Arms and legs are represented adjacent to the vermis over the intermediate cortex of the hemispheres (Figure 42-11A). Visual input from the superior colliculi and visual cortex is distributed to both vermal and paravermal portions of the posterior lobe (Figure 42-3). This early mapping was based on recordings of surface potentials, which reflect the predominant input and provide only a coarse representation of somatotopic connections. More refined mapping studies of the cerebellar cortex based on single-cell recordings reveal that input from a given peripheral site, such as a local area of skin, diverges to multiple discrete patches of granule cells, an arrangement called a fractured somatotopy (Figure 42-11B). Recent anatomical studies of primates show that the deep cerebellar nuclei are also organized somatotopically. They are arranged to receive projections from the two maps on the dorsal and ventral surfaces of the inter-mediate and lateral zones of the cerebellar cortex and project to the magnocellular red nucleus and primary motor cortex via the thalamus (Figure 42-12). The Spinocerebellum Modulates the Descending Motor Systems in the Brain Stem and Cerebral Cortex Purkinje neurons in the spinocerebellum project somatotopically to different deep nuclei that control various components of the descending motor pathways. Neurons in the vermis in both the anterior and posterior lobes send projections to the fastigial nucleus, which in turn projects bilaterally to the brain stem reticular formation and lateral vestibular nuclei. The latter areas project directly to the spinal cord (Figure 42-10). Axons of the fastigial nucleus also cross to the contralateral side and project to the area's primary motor cortex controlling proximal muscles via a synapse in the ventrolateral nucleus of the thalamus (Figure 42-12). Thus the medial region of the cerebellum controls mainly the cortical and brain stem components of the medial descending systems. This control affects primarily the head and neck and proximal parts of the limb, rather than the wrist and digits. It is therefore important for movements of the face, mouth, and neck and for balance and postural control during voluntary motor tasks. Purkinje neurons in the intermediate part of the cerebellar hemisphere project to the interposed nucleus (Figure 42-12). Some axons of this nucleus exit through the superior cerebellar peduncle and cross to the contralateral side to terminate in the magnocellular portion of the red nucleus, whose axons cross back and descend to the spinal cord. Other axons from the interposed nucleus continue rostrally and terminate in the ventrolateral nucleus of the thalamus. This cerebellar receiving area (in ventral lateral thalamus) is located posterior to the area that receives input from the basal ganglia (the ventral anterior nuclei) and anterior to the area recieving from the lemniscal sensory system (ventral posterior lateral nucleus) (see Figure 18-5). These thalamic neurons project to the limb control areas of the primary motor cortex. By acting on the neurons that give rise to the rubrospinal and corticospinal systems, the intermediate cerebellum focuses its action on limb muscles and axial musculature. Because the axons of the interposed nucleus cross to the contralateral side and the rubrospinal and corticospinal tracts cross back (Figure 42-12), cerebellar lesions can disrupt movements of ipsilateral limbs. Figure 19.4. Regions of the cerebral cortex that project to the cerebellum (shown in green). The cortical projections to the cerebellum are mainly from the sensory association cortex of the parietal lobe and motor association areas of the frontal lobe. Projections to the Cerebellum The cerebral cortex is by far the largest source of inputs to the cerebellum, the major destination being the cerebrocerebellum (see Figure 19.3 and Table 19.2). These pathways arise from a somewhat more circumscribed area of the cortex than do those to the basal ganglia (see Chapter 18). The majority originate in the primary motor and premotor cortices of the frontal lobe, the primary and secondary somatic sensory cortices of the anterior parietal lobe, and the secondary visual regions of the posterior parietal lobe (Figure 19.4). The visual input to the cerebellum originates mostly in association areas concerned with processing moving visual stimuli (i.e., the cortical targets of the magnocellular stream; see Chapter 12). Indeed, visually guided coordination of ongoing movement is one of the major tasks carried out by the cerebrocerebellum. Most of these cortical pathways relay in the pontine nuclei before entering thecerebellum (see Figure 19.3). There are also direct sensory inputs to the cerebellum (see Figure 19.3 and Table 19.2). Vestibular axons from the eighth cranial nerve and axons from the vestibular nuclei in the medulla project to the vestibulocerebellum. In addition, relay neurons in the dorsal nucleus of Clarke in the spinal cord (a group of relay neurons innervated by proprioceptive axons from the periphery; see Chapter 9) send their axons to the spinocerebellum. The vestibular and spinal inputs provide the cerebellum with information from the labyrinth in the ear, from muscle spindles, and from other mechanoreceptors that monitor the position and motion of the body. The somatic sensory input remains topographically mapped in the spinocerebellum such that there are orderly representations of the body surface within the cerebellum (Figure 19.5). These maps are “fractured,” however: Fine-grain electrophysiological analysis indicates that each small area of the body surface is represented multiple times by spatially separated clusters of cells rather than by a continuous topographic map of the body surface. The vestibular and spinal inputs remain ipsilateral from their point of entry in the brainstem, traveling in the inferior cerebellar peduncle (see Figure 19.3B). This arrangement indicates that, in contrast to most areas of the brain, the right cerebellum is concerned with the right half of the body and the leftcerebellum with the left half. Finally, the entire cerebellum receives modulatory inputs from the inferior olive and the locus ceruleus in the brainstem. These nuclei evidently participate in the learning and memory functions served by cerebellar circuitry (see p. 417).
- Figure 42-11 The spinocerebellum contains two somatotopic neural maps of the body. A. Two regions of the cerebellar surface contain somatotopic maps of the entire body. In both maps the head and trunk are located in the vermis, which also receives input from vestibular, visual, and auditory receptors. The limb representations are located on either side of the midline, in the intermediate part of the cerebellar hemispheres. B. Recordings of the receptive fields of granule cells in the rat cerebellar cortex reveal multiple representations of the same body parts in different locations, an arrangement referred to as fractured somatotopy. The receptive fields of individual granule cells are indicated by the red areas on body parts. (Adapted from Shambes et al. 1978.) The Spinocerebellum Regulates Body and Limb Movements Somatosensory Information Reaches the Spinocerebellum Through Direct and Indirect Mossy Fiber Pathways Cerebellar afferents from the spinal cord—mainly from somatosensory receptors—are distributed exclusively to the spinocerebellum (see Figure 42-3). Somatosensory information is conveyed to the spinocerebellum through several direct and indirect pathways.
- Figure 19.7. Summary diagram of motor modulation by the cerebrocerebellum. The central processing component, the cerebrocerebellar cortex, receives massive input from the cerebral cortex and generates signals that adjust the responses of upper motor neurons to regulate the course of a movement. Note that modulatory inputs also influence the processing of information within the cerebellar cortex. The output signals from the cerebellar cortex are relayed to the thalamus and then back to the motor cortex, where they modulate the motor commands.
- Figure 19.8. Neurons and circuits of the cerebellum. (A) Neuronal types in the cerebellar cortex. Note that the various neuron classes are found in distinct layers. (B) Diagram showing convergent inputs onto the Purkinje cell from parallel fibers and local circuit neurons [boxed region shown at higher magnification in (C)]. The output of the Purkinje cells is to the deep cerebellar nuclei. (C) Electron micrograph showing Purkinje cell dendritic shaft with three spines contacted by synapses from a trio of parallel fibers. (C courtesy of A.-S. La Mantia and P. Rakic.) Circuits within the Cerebellum The ultimate destination of the afferent pathways to the cerebellar cortex is a distinctive cell type called the Purkinje cell ( Figure 19.8 ). However, the input from the cerebral cortex to the Purkinje cells is quite indirect. Neurons in the pontine nuclei receive a projection from the cerebral cortex and then relay the information to the contralateral cerebellar cortex. The axons from the pontine nuclei and other sources are called mossy fibers because of the appearance of their synaptic terminals . Mossy fibers synapse on granule cells in the granule cell layer of the cerebellar cortex (see Figures 19.8 and 19.9 ). The cerebellar granule cells are widely held to be the most abundant class of neurons in the human brain. They give rise to specialized axons called parallel fibers that ascend to the molecular layer of the cerebellar cortex. The parallel fibers bifurcate in the molecular layer to form T-shaped branches that relay information via excitatory synapses onto the dendritic spines of the Purkinje cells. The Purkinje cells present the most striking histological feature of the cerebellum. Elaborate dendrites extend into the molecular layer from a single subjacent layer of these giant nerve cell bodies (called the Purkinje layer). Once in the molecular layer, the Purkinje cell dendrites branch extensively in a plane at right angles to the trajectory of the parallel fibers ( Figure 19.8A ). In this way, each Purkinje cell is in a position to receive input from a large number of parallel fibers, and each parallel fiber can contact a very large number of Purkinje cells (on the order of tens of thousands). The Purkinje cells receive a direct modulatory input on their dendritic shafts from the climbing fibers , all of which arise in the inferior olive ( Figure 19.8B ). Each Purkinje cell receives numerous synaptic contacts from a single climbing fiber. In most models of cerebellum function, the climbing fibers regulate movement by modulating the effectiveness of the mossy—parallel fiber connection with the Purkinje cells. The Purkinje cells project in turn to the deep cerebellar nuclei. They are the only output cells of the cerebellar cortex. Since the Purkinje cells are GABAergic, the output of the cerebellar cortex is wholly inhibitory. However, the deep cerebellar nuclei receive excitatory input from the collaterals of the mossy and climbing fibers. The Purkinje cell inhibition of the deep nuclei serves to modulate the level of this excitation ( Figure 19.9 ). Inputs from local circuit neurons modulate the inhibitory activity of Purkinje cells and occur on both dendritic shafts and the cell body. The most powerful of these local inputs are inhibitory complexes of synapses made around the Purkinje cell bodies by basket cells (see Figure 19.8A , B ). Another type of local circuit neuron, the stellate cell , receives input from the parallel fibers and provides an inhibitory input to the Purkinje cell dendrites. Finally, the molecular layer contains the apical dendrites of a cell type called Golgi cells ; these neurons have their cell bodies in the granular cell layer. The Golgi cells receive input from the parallel fibers and provide an inhibitory feedback to the cells of origin of the parallel fibers (the granule cells). This basic circuit is repeated over and over throughout every subdivision of the cerebellum in all mammals and is the fundamental functional module of thecerebellum. Modulation of signal flow through these modules provides the basis for both real-time regulation of movement and the long-term changes in regulation that underlie motor learning. The flow of signals through this admittedly complex intrinsic circuitry is best described in reference to the Purkinje cells (see Figure 19.9 ). The Purkinje cells receive two types of excitatory input from outside of the cerebellum, one directly from the climbing fibers and the other indirectly via the parallel fibers of the granule cells. The Golgi, stellate, and basket cells control the flow of information through the cerebellar cortex. For example, the Golgi cells form an inhibitory feedback that may limit the duration of the granule cell input to the Purkinje cells, whereas the basket cells provide lateral inhibition that may focus the spatial distribution of Purkinje cell activity. The Purkinje cells modulate the activity of the deep cerebellar nuclei, which are driven by the direct excitatory input they receive from the collaterals of the mossy and climbing fibers. The modulation of cerebellar output also occurs at the level of the Purkinje cells (see Figure 19.9 ). This latter modulation may be responsible for the motor learning aspect of cerebellar function. According to a model proposed by Masao Ito and his colleagues at Tokyo University, the climbing fibers relay the message of a motor error to the Purkinje cells. This message produces long-term reductions in the Purkinje cell responses to mossy-parallel fiber inputs. This inhibitory effect on the Purkinje cell responses disinhibits the deep cerebellar nuclei (for an account of the probable cellular mechanism for this long-term reduction in the efficacy of the parallel fiber synapse on Purkinje cells, see Chapter 25 ). As a result, the output of the cerebellum to the various sources of upper motor neurons is enhanced, in much the way that this process occurs in the basal ganglia (see Chapter 18 ).
- Figure 19.9. Excitatory and inhibitory connections in the cerebellar cortex and deep cerebellar nuclei. The excitatory input from mossy fibers and climbing fibers to Purkinje cells and deep nuclear cells is basically the same. Additional convergent input onto the Purkinje cell from local circuit neurons (basket and stellate cells) and other Purkinje cells establishes a basis for the comparison of ongoing movement and sensory feedback derived from it. The Purkinje cell output to the deep cerebellar nuclear cell thus generates an error correction signal that can modify movements already begun. The climbing fibers modify the efficacy of the parallel fiber-Purkinje cell connection, producing long-term changes in cerebellar output. (After Stein, 1986.) Cerebellar Circuitry and the Coordination of Ongoing Movement As expected for a structure that monitors and regulates motor behavior, neuronal activity in the cerebellum changes continually during the course of amovement. For instance, the execution of a relatively simple task like flipping the wrist back and forth elicits a dynamic pattern of activity in both the Purkinje cells and the deep cerebellar nuclear cells that closely follows the ongoing movement (Figure 19.10). Both types of cells are tonically active at rest and change their frequency of firing as movements occur. The neurons respond selectively to various aspects of movement, including extension or contraction of specific muscles, the position of the joints, and the direction of the next movement that will occur. All this information is therefore encoded by changes in the firing frequency of Purkinje cells and deep cerebellar nuclear cells. As these neuronal response properties predict, cerebellar lesions and disease tend to disrupt the modulation and coordination of ongoing movements. Thus, the hallmark of patients with cerebellar damage is difficulty producing smooth, well-coordinated movements. Instead, movements tend to be jerky and imprecise, a pattern referred to as cerebellar ataxia (Box A). Many of these difficulties in performing movements can be explained as disruption of the cerebellum's role in correcting errors in ongoing movements. Normally, the cerebellar error correction mechanism ensures that movements are modified to cope with changing circumstances. As described earlier, the Purkinje cells and the deep cerebellar nuclear cells recognize potential errors by comparing patterns of convergent activity that are concurrently available to both cell types; the deep nuclear cells then send corrective signals to the upper motor neurons in order to maintain or improve the accuracy of the movement. As in the case of the basal ganglia, studies of the oculomotor system, saccades in particular, have contributed greatly to understanding the contribution that the cerebellum makes to motor error reduction. For example, cutting part of the tendon to the lateral rectus muscles in one eye of a monkey weakens horizontal eye movements by that eye (Figure 19.11). When a patch is then placed over the normal eye to force the animal to use its weak eye, the saccades performed by the weak eye are hypometric ; as expected, they fall short of visual targets. Then, over the next few days, the amplitude of the saccades gradually increases until they again become accurate. If the patch is then switched to cover the weakened eye, the saccades performed by the normal eye are hypermetric . In other words, over a period of a few days the nervous system corrects the error in the saccades made by the weak eye by increasing the gain in the saccade motor system. Lesions in the vermis of the spinocerebellum (see Figure 19.1) eliminate this ability to reduce the motor error. Similar evidence of the cerebellar contribution to movement has come from studies of the vestibulo-ocular reflex (VOR) in monkeys and humans. The VOR works to keep the eyes trained on a visual target during head movements (see Chapter 14). The relative simplicity of this reflex has made it possible to analyze some of the mechanisms that enable motor learning as a process of error reduction. When a visual image on the retina shifts its position as a result of head movement, the eyes must move at the same velocity in the opposite direction to maintain a stable percept. In these studies, the adaptability of the VOR to changes in the nature of incoming sensory information is challenged by fitting subjects (either monkeys or humans) with magnifying or minifying spectacles (Figure 19.12). Because the glasses alter the size of the visual image on the retina, the compensatory eye movements, which would normally have maintained a stable image of an object on the retina, are either too large or too small. Over time, subjects (whether monkeys or humans) learn to adjust the distance the eyes must move in response to head movements to accord with the artificially altered size of the visual field. Moreover, this change is retained for significant periods after the spectacles are removed and can be detected electrophysiologically in recordings from cerebellar Purkinje cells and neurons in the deepcerebellar nuclei. Information that reflects this change in the sensory context of the VOR must therefore be learned and remembered to eliminate the artificially introduced error. Once again, if the cerebellum is damaged or removed, the ability of the VOR to adapt to the new conditions is lost. These observations support the conclusion that the cerebellum is critically important in error reduction during motor learning. Cerebellar circuitry also provides real-time error correction during ongoing movements. This function is accomplished by changes in the tonically inhibitory activity of Purkinje cells that in turn influence the tonically excitatory deep cerebellar nuclear cells. The resulting effects on the ongoing activity of the deepcerebellar nuclear cells adjust the cerebellar output signals to the upper motor neurons in the cortex and brainstem Summary Two sets of upper motor neuron pathways make distinct contributions to the control of the local circuitry in the brainstem and spinal cord. One set originates from neurons in brainstem centers—primarily the reticular formation and the vestibular nuclei—and is responsible for postural regulation. The reticular formation is especially important in feedforward control of posture (that is, movements that occur in anticipation of changes in body stability). In contrast, the neurons in the vestibular nuclei that project to the spinal cord are especially important in feedback postural mechanisms (i.e., in producing movements that are generated in response to sensory signals that indicate an existing postural disturbance). The other major upper motor neuronpathway originates from the frontal lobe and includes projections from the primary motor cortex and the nearby premotor areas. The premotor cortices are responsible for planning and selecting movements, whereas the primary motor cortex is responsible for their execution. The motor cortex influences movements directly by contacting lower motor neurons and local circuit neurons in the spinal cord and brainstem, and indirectly by innervating neurons in brainstem centers (in this case, the reticular formation and red nucleus) that in turn project to lower motor neurons and circuits. Although the brainstem pathways can independently organize gross motor control, direct projections from the motor cortex to local circuit neurons in the brainstem and spinal cord are essential for the fine, fractionated movements of the distal parts of the limbs, the tongue, and face that are especially important in our daily lives.
- The motor cortex also includes Area 6, which lies rostrally to Area 4 and is divided into the premotor area (or premotor cortex) and the supplementary motor area. The premotor cortex is believed to help regulate posture by dictating an optimal position to the motor cortex for any given movement. The supplementary motor area, for its part, seems to influence the planning and initiation of movements on the basis of past experience. The mere anticipation of a movement triggers neural transmissions in the supplementary motor area.Besides the frontal cortex, the posterior parietal cortex clearly plays a role in voluntary movements, by assessing the context in which they are being made. The parietal cortex receives somatosensory, proprioreceptive, and visual inputs, then uses them to determine such things as the positions of the body and the target in space. It thereby produces internal models of the movement to be made, prior to the involvement of the premotor and motor cortices. Within the posterior parietal cortex, two particular areas are distinguished. Area 5 receives information from somatosensory areas 1, 2, and 3 of the cortex. Area 7 further integrates the already highly integrated signals from the visual areas of the cortex, such as MT and V5. The parietal lobes are themselves closely interconnected with the prefrontal areas , and together these two regions represent the highest level of integration in the motor control hierarchy. It is here that the decisions are made about what action to take. The posterior parietal and prefrontal areas send their axons to Area 6 which, once it has been informed about the kind of action to take, helps to determine the characteristics of the appropriate movement for this purpose.
- Figure 17.9. Topographic map of the body musculature in the primary motor cortex. (A) Location of primary motor cortex in the precentral gyrus. (B) Section along the precentral gyrus, illustrating the somatotopic organization of the motor cortex. The most medial parts of the motor cortex are responsible for controlling muscles in the legs; the most lateral portions are responsible for controlling muscles in the face. (C) Dispro- portional representation of various portions of the body musculature in the motor cortex. Representations of parts of the body that exhibit fine motor control capabilities (such as the hands and face) occupy a greater amount of space than those that exhibit less precise motor control (such as the trunk). In 1870, Hitzig and Fritsch electrically stimulated various parts of a dog's motor cortex. They observed that depending on what part of the cortex they stimulated, a different part of the body contracted. Then they found that if they destroyed this same small area of the cortex, the corresponding part of the body became paralyzed. This is how it was discovered that every part of the body has a particular region of the primary motor cortex that controls its movement. But what is remarkable about this "motor map" is that certain parts of the body—those that can make the finest movements—take up much more space than others. These parts of the body are shown larger than the others in the illustration here. Dr. Penfield's during 1930 experiments in stimulating the cortex enabled him to develop a complete map of the motor cortex, known as the motor homunculus (there are also other kinds, such as the sensory homunculus). The most striking aspect of this map is that the areas assigned to various body parts on the cortex are proportional not to their size, but rather to the complexity of the movements that they can perform. Hence, the areas for the hand and face are especially large compared with those for the rest of the body. This is no surprise, because the speed and dexterity of human hand and mouth movements are precisely what give us two of our most distinctly human faculties: the ability to use tools and the ability to speak. Functional Organization of the Primary Motor Cortex Clinical observations and experimental work dating back a hundred years or more have provided a reasonably coherent picture of the functional organization of the motor cortex. By the end of the nineteenth century, experimental work in animals by the German physiologists G. Theodor Fritsch and Eduard Hitzig had shown that electrical stimulation of the motor cortex elicits contractions of muscles on the contralateral side of the body. At about the same time, the British neurologist John Hughlings Jackson surmised that the motor cortex contains a complete representation, or map, of the body's musculature. Jackson reached this conclusion from the fact that the abnormal movements during some types of epileptic seizures “march” systematically from one part of the body to another. For instance, partial motor seizures may start with abnormal movements of a finger, progress to involve the entire hand, then the forearm, the arm, the shoulder, and, finally, the face. This early evidence for motor maps in the cortex was confirmed shortly after the turn of the nineteenth century when Charles Sherrington published his classical studies of the organization of the motor cortex in great apes, using focal electrical stimulation. During the 1930s, one of Sherrington's students, the American neurosurgeon Wilder Penfield, extended this work by demonstrating that the human motor cortex also contains a spatial map of the body's musculature. By correlating the location of muscle contractions with the site of electrical stimulation on the surface of the motor cortex (the same method used by Sherrington), Penfield mapped the representation of the muscles in the precentral gyrus in over 400 neurosurgical patients (Figure 17.9). He found that this motor map shows the same disproportions observed in the somatic sensory maps in the postcentral gyrus (see Chapter 9). Thus, the musculature used in tasks requiring fine motor control (such as movements of the face and hands) occupies a greater amount of space in the map than does the musculature requiring less precise motor control (such as that of the trunk). The behavioral implications of cortical motor maps are considered in Boxes B and C The introduction in the 1960s of intracortical microstimulation (a more refined method of cortical activation) allowed a more detailed understanding of motormaps. Microstimulation entails the delivery of electrical currents an order of magnitude smaller than those used by Sherrington and Penfield. By passing the current through the sharpened tip of a metal microelectrode inserted into the cortex, the upper motor neurons in layer V that project to lower motor neuroncircuitry can be stimulated focally. Although intracortical stimulation generally confirmed Penfield's spatial map in the motor cortex, it also showed that the finer organization of the map is rather different than most neuroscientists imagined. For example, when microstimulation was combined with recordings of muscle electrical activity, even the smallest currents capable of eliciting a response initiated the excitation of several muscles (and the simultaneous inhibition of others), suggesting that organized movements rather than individual muscles are represented in the map (see Box B). Furthermore, within major subdivisions of the map (e.g., arm, forearm, or finger regions), a particular movement could be elicited by stimulation of widely separated sites, indicating that nearby regions are linked by local circuits to organize specific movements. This interpretation has been supported by the observation that the regions responsible for initiating particular movements overlap substantially. What Do Motor Maps Represent? Electrical stimulation studies carried out by the neurosurgeon Wilder Penfield and his colleagues in human patients (and by Clinton Woolsey and his colleagues in experimental animals) clearly demonstrated a systematic map of the body's musculature in the primary motor cortex (see text). The fine structure of this map, however, has been a continuing source of controversy. Is the map in the motor cortex a “piano keyboard” for the control of individual muscles, or is it a map of movements, in which specific sites control multiple muscle groups that contribute to the generation of particular actions? Initial experiments implied that the map in the motor cortex is a fine-scale representation of individual muscles. Thus, stimulation of small regions of the map activated single muscles, suggesting that vertical columns of cells in the motor cortex were responsible for controlling the actions of particular muscles, much as columns in the somatic sensory map are thought to analyze particular types of stimulus information (see Chapter 9). More recent studies using anatomical and physiological techniques, however, have shown that the map in the motor cortex is far more complex than a columnar representation of particular muscles. Individual pyramidal tract axons are now known to terminate on sets of spinal motor neurons that innervate different muscles. This relationship is evident even for neurons in the hand representation of the motor cortex, the region that controls the most discrete, fractionated movements. Furthermore, cortical microstimulation experiments have shown that a single muscle is represented multiple times over a wide region of the motor cortex (about 2–3 mm in primates) in a complex, mosaic fashion. It seems likely that horizontal connections within the motor cortex create ensembles of neurons that coordinate the pattern of firing in the population of ventral horn cells that ultimately generate a given movement. Thus, while the somatotopic maps in the motor cortex generated by early studies are correct in their overall topography, the fine structure of the map is far more intricate. Unraveling these details of motor maps still holds the key to understanding how patterns of activity in the motor cortex generate a given movement.
- Figure 38-4 The major inputs to the motor cortex in monkeys. A. The major inputs to the primary motor cortex. (PMd = dorsal premotor area; PMv = ventral premotor area; S1 = primary sensory cortex; SMA = supplementary motor area.) B. The major inputs to the premotor areas. Dense interconnections between the premotor areas are not shown here. Each Cortical Motor Area Receives Unique Cortical and Subcortical Inputs The primary motor cortex receives somatotopically organized inputs directly from two sources. First, it receives inputs from the primary somatosensory cortex (areas 1, 2, and 3). This means that, like neurons in somatosensory cortex, neurons in the motor cortex have receptive fields in the periphery. For example, some neurons in the motor cortex receive proprioceptive input from the muscles to which they project and many neurons in the hand region of the motor map respond to tactile stimuli applied to specific regions of the digits and palms. These so-called transcortical circuits are discussed later. Second, the primary motor cortex receives inputs from posterior parietal area 5. Posterior parietal areas 5 and 7 are involved in integrating multiple sensory modalities for motor planning (Figure 38-4A). The premotor areas receive major inputs from areas 5 and 7 as well as from area 46 in the prefrontal cortex (Figure 38-4B). Each premotor area has its own pattern of inputs from distinct locations in areas 5 and 7. Area 46 projects mainly to the ventral premotor area and is important in working memory; it is thought to store information about the location of objects in space only long enough to guide a movement. There are also dense connections between the premotor areas themselves. These connections are thought to allow working memory to influence specific aspects of motor planning that are mediated by the different premotor subregions.
- Figure 38-5 The motor cortex receives inputs from the cerebellum via the thalamus. VLo and VLc = oral (rostral) and caudal portions of the ventrolateral nucleus; VPLo = oral portion of the ventral posterolateral nucleus; X = nucleus X. The premotor areas and primary motor cortex also receive input from the basal ganglia and cerebellum via different sets of nuclei in the ventrolateral thalamus (Figure 38-5). The basal ganglia and cerebellum do not project directly to the spinal cord. An important feature of the relationship between cortical areas and subcortical structures is the reciprocal nature of their connections. Each cortical motor area appears to have a unique pattern of cortical and subcortical input. Thus there are many cortico-subcortical loops, each one making a different contribution to a motor behavior (Chapter 43).
- F igure 17.8. The corticospinal tract. Neurons in the motor cortex give rise to axons that travel through the internal capsule and coalesce on the ventral surface of the midbrain, within the cerebral peduncle. These axons continue through the pons and come to lie on the ventral surface of the medulla, giving rise to the pyramids. Most of these pyramidal fibers cross in the caudal part of the medulla to form the lateral corticospinal tract in the spinal cord. Those axons that do not cross (not illustrated) descend on the same side and form the ventral corticospinal tract (see Figure 17.6 ). The axons that terminate in the reticular formation of the pons and medulla comprise components of the corticobulbar tract. Transmission of Signals from the Motor Cortex to the Muscles Motor signals are transmitted directly from the cortex to the spinal cord through the corticospinal tract and indirectly through multiple accessory pathways that involve the basal ganglia, cerebellum, and various nuclei of the brain stem. In general, the direct pathways are concerned more with discrete and detailed movements, especially of the distal segments of the limbs, particularly the hands and fingers. Corticospinal (Pyramidal) Tract The most important output pathway from the motor cortex is the corticospinal tract, also called the pyramidal tract, shown in Figure 55–4.The corticospinal tract originates about 30 per cent from the primary motor cortex, 30 per cent from the premotor and supplementary motor areas, and 40 per cent from the somatosensory areas posterior to the central sulcus. After leaving the cortex, it passes through the posterior limb of the internal capsule (between the caudate nucleus and the putamen of the basal ganglia) and then downward through the brain stem, forming the pyramids of the medulla. The majority of the pyramidal fibers then cross in the lower medulla to the opposite side and descend into the lateral corticospinal tracts of the cord, finally terminating principally on the interneurons in the intermediate regions of the cord gray matter; a few terminate on sensory relay neurons in the dorsal horn, and a very few terminate directly on the anterior motor neurons that cause muscle contraction. A few of the fibers do not cross to the opposite side in the medulla but pass ipsilaterally down the cord in the ventral corticospinal tracts. Many if not most of these fibers eventually cross to the opposite side of the cord either in the neck or in the upper thoracic region. These fibers may be concerned with control of bilateral postural movements by the supplementary motor cortex. The most impressive fibers in the pyramidal tract are a population of large myelinated fibers with a mean diameter of 16 micrometers. These fibers originate from giant pyramidal cells, called Betz cells, that are found only in the primary motor cortex. The Betz cells are about 60 micrometers in diameter, and their fibers transmit nerve impulses to the spinal cord at a velocity of about 70 m/sec, the most rapid rate of transmission of any signals from the brain to the cord. There are about 34,000 of these large Betz cell fibers in each corticospinal tract. The total number of fibers in each corticospinal tract is more than 1 million, so these large fibers represent only 3 per cent of the total. The other 97 per cent are mainly fibers smaller than 4 micrometers in diameter that conduct background tonic signals to the motor areas of the cord. Other Fiber Pathways from the Motor Cortex The motor cortex gives rise to large numbers of additional, mainly small fibers that go to deep regions in the cerebrum and brain stem, including the following: 1. The axons from the giant Betz cells send short collaterals back to the cortex itself. These collaterals are believed to inhibit adjacent regions of the cortex when the Betz cells discharge, thereby “sharpening” the boundaries of the excitatory signal. 2. A large number of fibers pass from the motor cortex into the caudate nucleus and putamen. From there, additional pathways extend into the brain stem and spinal cord, as discussed in the next chapter, mainly to control body postural muscle contractions. 3. A moderate number of motor fibers pass to red nuclei of the midbrain . From these, additional fibers pass down the cord through the rubrospinal tract. 4. A moderate number of motor fibers deviate into the reticular substance and vestibular nuclei of the brain stem; from there, signals go to the cord by way of reticulospinal and vestibulospinal tracts, and others go to the cerebellum by way of reticulocerebellar and vestibulocerebellar tracts. 5. A tremendous number of motor fibers synapse in the pontile nuclei, which give rise to the pontocerebellar fibers, carrying signals into the cerebellar hemispheres. 6. Collaterals also terminate in the inferior olivary nuclei, and from there, secondary olivocerebellar fibers transmit signals to multiple areas of the cerebellum. Thus, the basal ganglia, brain stem, and cerebellum all receive strong motor signals from the corticospinal system every time a signal is transmitted down the spinal cord to cause a motor activity.
- Excitation of the Spinal Cord Motor Control Areas by the Primary Motor Cortex and Red Nucleus Vertical Columnar Arrangement of the Neurons in the Motor Cortex. In Chapters 47 and 51, we pointed out that the cells in the somatosensory cortex and visual cortex are organized in vertical columns of cells . In like manner, the cells of the motor cortex are organized in vertical columns a fraction of a millimeter in diameter, with thousands of neurons in each column. Each column of cells functions as a unit, usually stimulating a group of synergistic muscles, but sometimes stimulating just a single muscle. Also, each column has six distinct layers of cells, as is true throughout nearly all the cerebral cortex. The pyramidal cells that give rise to the corticospinal fibers all lie in the fifth layer of cells from the cortical surface. Conversely, the input signals all enter by way of layers 2 through 4. And the sixth layer gives rise mainly to fibers that communicate with other regions of the cerebral cortex itself. Function of Each Column of Neurons. The neurons of each column operate as an integrative processing system, using information from multiple input sources Motor cortex Interpositus nucleus Dentate nucleus Cerebellum Red nucleus Reticular formation Rubrospinal tract Corticorubral tract Figure 55–5 Corticorubrospinal pathway for motor control, showing also the relation of this pathway to the cerebellum. The Nervous System: C. Motor and Integrative Neurophysiology to determine the output response from the column. In addition, each column can function as an amplifying system to stimulate large numbers of pyramidal fibers to the same muscle or to synergistic muscles simultaneously. This is important, because stimulation of a single pyramidal cell can seldom excite a muscle. Usually, 50 to 100 pyramidal cells need to be excited simultaneously or in rapid succession to achieve definitive muscle contraction. Dynamic and Static Signals Transmitted by the Pyramidal Neurons. If a strong signal is sent to a muscle to cause initial rapid contraction, then a much weaker continuing signal can maintain the contraction for long periods thereafter. This is the usual manner in which excitation is provided to cause muscle contractions. To do this, each column of cells excites two populations of pyramidal cell neurons, one called dynamic neurons and the other static neurons. The dynamic neurons are excessively excited for a short period at the beginning of a contraction, causing the initial rapid development of force. Then the static neurons fire at a much slower rate, but they continue firing at this slow rate to maintain the force of contraction as long as the contraction is required. The neurons of the red nucleus have similar dynamic and static characteristics, except that a greater percentage of dynamic neurons is in the red nucleus and a greater percentage of static neurons is in the primary motor cortex. This may be related to the fact that the red nucleus is closely allied with the cerebellum, and the cerebellum plays an important role in rapid initiation of muscle contraction, as explained in the next chapter. Somatosensory Feedback to the Motor Cortex Helps Control the Precision of Muscle Contraction When nerve signals from the motor cortex cause a muscle to contract, somatosensory signals return all the way from the activated region of the body to the neurons in the motor cortex that are initiating the action. Most of these somatosensory signals arise in (1) the muscle spindles, (2) the tendon organs of themuscle tendons, or (3) the tactile receptors of the skin overlying the muscles. These somatic signals often cause positive feedback enhancement of the muscle contraction in the following ways: In the case of the muscle spindles, if the fusimotor muscle fibers in the spindles contract more than the large skeletal muscle fibers contract, the central portions of the spindles become stretched and, therefore, excited. Signals from these spindles then return rapidly to the pyramidal cells in the motor cortex to signal them that the large muscle fibers have not contracted enough.The pyramidal cells further excite the muscle, helping its contraction to catch up with the contraction of the muscle spindles. In the case of the tactile receptors, if the muscle contraction causes compression of the skin against an object, such as compression of the fingers around an object being grasped, the signals from the skin receptors can, if necessary, cause further excitation of the muscles and, therefore, increase the tightness of the hand grasp. Stimulation of the Spinal Motor Neurons Figure 55–6 shows a cross section of a spinal cord segment demonstrating (1) multiple motor and sensorimotor control tracts entering the cord segment and (2) a representative anterior motor neuron in the middle of the anterior horn gray matter. The corticospinal tract and the rubrospinal tract lie in the dorsal portions of the lateral white columns. Their fibers terminate mainly on interneurons in the intermediate area of the cord gray matter. In the cervical enlargement of the cord where the hands and fingers are represented, large numbers of both corticospinal and rubrospinal fibers also terminate directly on the anterior motor neurons, thus allowing a direct route from the brain to activate muscle contraction.This is in keeping with the fact that the primary motor cortex has an extremely high degree of representation for fine control of hand, finger, and thumb actions. Patterns of Movement Elicited by Spinal Cord Centers. From Chapter 54, recall that the spinal cord can provide certain specific reflex patterns of movement in response to sensory nerve stimulation. Many of these same patterns are also important when the cord’s anterior motor neurons are excited by signals from the brain. For example, the stretch reflex is functional at all times, helping to damp any oscillations of the motor movements initiated from the brain, and probably also providing at least part of the motive power required to cause muscle contractions when the intrafusal fibers of the muscle spindles contract more than the large skeletal muscle fibers do, thus eliciting reflex “servoassist” stimulation of the muscle, in addition to the direct stimulation by the corticospinal fibers. Also, when a brain signal excites a muscle, it usually is not necessary to transmit an inverse signal to relax the antagonist muscle at the same time; this is achieved by the reciprocal innervation circuit that is always present in the cord for coordinating the function of antagonistic pairs of muscles. Finally, other cord reflex mechanisms, such as withdrawal, stepping and walking, scratching, and postural mechanisms, can each be activated by “ command” signals from the brain. Thus, simple command signals from the brain can initiate many normal motor activities, particularly for such functions as walking and attaining different postural attitudes of the body.
- Figure 17.10. Experimental apparatus developed to record the activity of single neurons in awake primates trained to perform specific movements. About the same time that these studies were being undertaken, Ed Evarts and his colleagues at the National Institutes of Health were pioneering a technique in which implanted microelectrodes were used to record the electrical activity of individual motor neurons in awake, behaving monkeys (Figure 17.10). In these experiments, the monkeys were trained to perform a variety of motor tasks, thus providing a means of correlating neuronal activity with voluntary movements. Evarts and his group found that the force generated by contracting muscles changed as a function of the firing rate of upper motor neurons . Moreover, the firing rates of the active neurons often changed prior to movements involving very small forces. Evarts therefore proposed that the primary motor cortex contributes to the initial phase of recruitment of lower motor neurons involved in the generation of finely controlled movements. Additional experiments showed that the activity of primary motor neurons is correlated not only with the magnitude, but also with the direction of the force produced by muscles. Thus, some neurons show progressively less activity as the direction of movement deviates from the neuron's “preferred direction.” The early mapping experiments stimulating the cortical surface electrically (and more recently magnetically) initially led to the simplistic idea that the primary motor cortex acts as a massive switchboard with a switch controlling individual muscles or small groups of adjacent muscles. More detailed studies, however, using microelectrodes inserted into the depths of the cortex (intracortical microstimulation or ICMS) to stimulate small groups of output neurons indicate that this simple view is incorrect. Whereas the weakest stimuli may evoke the contraction of individual muscles, the same muscles are invariably activated from several separate sites as well, indicating that neurons in several cortical sites project axons to the same target (Figure 38-3). In addition, most stimuli activate several muscles, with muscles rarely being activated individually. This is corroborated by recent anatomical and physiological experiments showing that the terminal distributions of individual corticospinal axons diverge to motor neurons innervating more than one muscle. Instead of a simple switchboard of muscle representations, detailed maps of monkey motor cortex suggest a concentric organization: sites influencing distal muscles are contained at the center of a wider area containing sites that also influence more proximal muscles, while sites in the peripheral ring around this central area influence proximal muscles alone. An implication of this redundancy in muscle representation is that inputs to motor cortex from other cortical areas can combine proximal and distal muscles in different ways in different tasks.
- Figure 38-8 Direct corticospinal control of motor neurons is necessary for fine control of the digits. A. A monkey is able to pick up a food morsel from a small well using the index finger and thumb. B. After bilateral sectioning of the pyramidal tract the monkey can only remove food from the well by grabbing with the whole hand. (From Lawrence DG, Kuypers HGJM. 1968. The functional organization of the motor system in the monkey. Brain XCI.) Corticospinal Axons Influence Spinal Motor Neurons Through Direct and Indirect Connections Corticospinal neurons make powerful and direct excitatory connections with alpha motor neurons in the spinal cord. A unique feature of the corticospinal synapse is that successive cortical stimuli produce progressively larger excitatory postsynaptic potentials in spinal motor neurons. This potentiating connection is one of the mechanisms that permit monkeys to perform individual movements of the digits, including the grasping of small objects (Figure 38-8A) and to isolate movement of proximal joints. This ability is lost permanently after sectioning the pyramidal tracts in the medulla (Figure 38-8B) or after ablating the hand-control area of the motor cortex. Corticospinal fibers also terminate on interneurons in the spinal cord, which in turn project to alpha motor neurons. These indirect connections with motor neurons regulate a larger number of muscles than do the direct connections and so may contribute to the organization of multijointed movements such as reaching and walking. Sectioning the medullary pyramidal tracts, which interrupts the projection of corticospinal axons from the primary motor cortex and premotor areas, produces contralateral weakness in monkeys. But the animals recover after a period of months, leaving only deficits in speed of movement and in the rate of force development. These deficits can be attributed to interruption of the projections from the primary motor cortex because similar deficits arise from lesions in primary motor cortex but not from lesions in premotor areas. Animals with pyramidal tract lesions climb, jump, and appear generally normal. Their partial recovery is possible because cortical commands have indirect access to spinal motor neurons through the descending systems of the brain stem (Chapter 33). Nevertheless, individuated movements of the digits are lost permanently, and the wrist, elbow, and shoulder become linked in extensor or flexor synergies. Corticospinal projections also have inhibitory effects on spinal motor neurons. Direct recordings in monkeys and indirect evidence from reflex testing in humans indicate that corticospinal inhibition is mediated by the Ia inhibitory interneuron, the same interneuron responsible for the reciprocal inhibition of stretch reflexes (Figure 38-9). Because these spinal interneurons receive peripheral inputs and are able to respond directly to ongoing changes in somatic sensory input, the higher centers of the brain are freed from the need to manage all the details of movements and instead can use the spinal circuits as components of more complex behaviors, much like the subroutines of a computer program. Effect of Lesions in the Motor Cortex or in the Corticospinal Pathway—The “Stroke” The motor control system can be damaged by the common abnormality called a “stroke.” This is caused either by a ruptured blood vessel that hemorrhages into the brain or by thrombosis of one of the major arteries supplying the brain. In either case, the result is loss of blood supply to the cortex or to the corticospinal tract where it passes through the internal capsule between the caudate nucleus and the putamen.Also, experiments have been performed in animals to selectively remove different parts of the motor cortex. Removal of the Primary Motor Cortex (Area Pyramidalis). Removal of a portion of the primary motor cortex—the area that contains the giant Betz pyramidal cells— causes varying degrees of paralysis of the represented muscles. If the sublying caudate nucleus and adjacent premotor and supplementary motor areas are not damaged, gross postural and limb “fixation” movements can still occur, but there is loss of voluntary control of discrete movements of the distal segments of the limbs, especially of the hands and fingers. This does not mean that the hand and finger muscles themselves cannot contract; rather, the ability to control the fine movements is gone. From these observations, one can conclude that the area pyramidalis is essential for voluntary initiation of finely controlled movements, especially of the hands and fingers. Muscle Spasticity Caused by Lesions That Damage Large Areas Adjacent to the Motor Cortex. The primary motor cortex normally exerts a continual tonic stimulatory effect on the motor neurons of the spinal cord; when this stimulatory effect is removed, hypotonia results. Most lesions of the motor cortex, especially those caused by a stroke , involve not only the primary motor cortex but also adjacent parts of the brain such as the basal ganglia. In these instances, muscle spasm almost invariably occurs in the afflicted muscle areas on the opposite side of the body (because the motor pathways cross to the opposite side). This spasm results mainly from damage to accessory pathways from the nonpyramidal portions of the motor cortex. These pathways normally inhibit the vestibular and reticular brain stem motor nuclei.When these nuclei cease their state of inhibition (i.e., are “disinhibited”), they become spontaneously active and cause excessive spastic tone in the involved muscles, as we discuss more fully later in the chapter. This is the spasticity that normally accompanies a “stroke” in a human being.
- Figure 17.12. Directional tuning of an upper motor neuron in the primary motor cortex. (A) A monkey is trained to move a joystick in the direction indicated by a light. (B) The activity of a single neuron was recorded during arm movements in each of eight different directions (zero indicates the time of movement onset, and each short vertical line in this raster plot represents an action potential). The activity of the neuron increased before movements between 90 and 225 degrees (yellow zone), but decreased in anticipation of movements between 0 and 315 degrees (purple zone). (C) Plot showing that the neuron's discharge rate was greatest before movements in a particular direction, which defines the neuron's “preferred direction.” (D) The black lines indicate the discharge rate of individual upper motor neurons prior to each direction of movement. By combining the responses of all the neurons, a “population vector” can be derived that represents the movement direction encoded by the simultaneous activity of the entire population. (After Georgeopoulos et al., 1986.) Finally, the relative amount of activity across large populations of neurons appears to encode the direction of visually-guided movements. Thus, the direction of movements in monkeys could be predicted by calculating a “neuronal population vector” derived simultaneously from the discharges of many “broadly tuned” upper motor neurons (Figure 17.12). These observations showed that the discharges of individual upper motor neurons cannot specify the direction of an arm movement, simply because they are tuned too broadly; rather, each arm movement must be encoded by the concurrent discharges of a large population of such neurons
- Figure 38-10 Activity in a corticospinal axon correlates with the direction and amplitude of muscle force rather than the direction of displacement. Records shown here were made while a monkey flexed its wrist under three load conditions. In each set of traces the top trace indicates the activity in a corticospinal neuron and the bottom trace wrist position, with upward deviation being flexion. When no load was applied (A) the neuron fired before and during flexion. When a load-opposing flexion was applied, activity in the neuron increased (B). When a load-assisting flexion was applied, the neuron fell silent (C). In all three conditions the wrist displacement was the same but the neuronal activity changed as the load changed. Thus the firing of the corticospinal neuron in this experiment is related to the force exerted during a movement and not to the displacement of the wrist. (From Evarts 1968.) The Primary Motor Cortex Executes Movements and Adapts Them to New Conditions Activity in Individual Neurons of the Primary Motor Cortex Is Related to Muscle Force To understand how cortical motor areas contribute to movement it is necessary to study how individual neurons are modulated in natural motor behaviors. This became possible in the 1960s when Edward Evarts succeeded in correlating the activity of single neurons with specific motor behaviors in active monkeys. Evarts found that activity in individual neurons in the primary motor cortex is modulated when monkeys either flex or extend the individual joints of their contralateral limbs. Individual neurons are maximally activated during movement of a particular joint and particular direction of movement. The changes in neuronal activity begin some 100 ms or more before the onset of movement. In a classic experiment Evarts showed that, during wrist flexion, the firing of primary motor cortex neurons varied with the amount of force the animal had to exert to move its hand, not with the amplitude of the hand's displacement (Figure 38-10). The activity of these cortical neurons therefore appears to signal the direction and amplitude of muscle force required to produce a movement rather than the actual displacement of the joint. Jun Tanji and Evarts found another, more surprising property of some primary motor cortex neurons. In these cells the baseline discharge changed while the animal waited for a signal to move in a predetermined direction. For example, a cell would change its level of baseline activity when a green light instructed the animal that an extension movement was to be made at a later signal (an instructed delay task). This pattern of activity was termed set related because it reflected the animal's preparation—or preparatory set —to respond to a later stimulus. These discharges demonstrated that the intent to perform a movement alters the firing pattern of neurons in the primary motor cortex hundreds of milliseconds before the movement takes place. Simple correlations of neuronal activity and behavior do not prove causality. Movement or set-related neurons might be concerned with early changes in postural muscle activity or some other process, rather than with the voluntary movement. The most common (and often the only possible) approach to relating neuronal activity to a specific behavior is to exclude confounding sources of correlation. However, in the case of primary motor cortex neurons, what is really needed is a way to know for sure whether activity that precedes a voluntary movement directly influences the muscles used in the movement. Only after a direct influence has been established can the relationship of these cells' activity to specific aspects of the movement be addressed meaningfully .
- Figure 17.11. The influence of single cortical upper motor neurons on muscle activity. (A) Diagram illustrates the spike triggering average method for correlating muscle activity with the discharges of single upper motor neurons. (B) The response of a thumb muscle (bottom trace) follows by a fixed latency the single spike discharge of a pyramidal tract neuron (top trace). This technique can be used to determine all the muscles that are influenced by a given motor neuron (see text). (After Porter and Lemon, 1993.) A further advance was made in the mid-1970s by the introduction of spike-triggered averaging (Figure 17.11). By correlating the timing of the cortical neuron's discharges with the onset times of the contractions generated by the various muscles used in a movement, this method provides a way of measuring the influence of a single cortical motor neuron on a population of lower motor neurons in the spinal cord. In recording such activity from different muscles as monkeys performed wrist flexion or extension, it became apparent that the activity of a number of different muscles is directly facilitated by the discharges of a given upper motor neuron. This peripheral muscle group was called the “muscle field” of the upper motor neuron. On average, the size of the muscle field in the wrist region was two to three muscles per upper motor neuron. These observations confirmed that single upper motor neurons contact several lowermotor neuron pools; the results are also consistent with the general conclusion that movements , rather than individual muscles, are encoded by the activity of the upper motor neurons in the cortex (see Box B). A major advance in this direction was made in the mid-1970s by Eberhard Fetz and co-workers, who used the spike-triggered averaging technique (Box 38-1) to identify neurons in the primary motor cortex that project directly to motor neurons, called corticomotoneuronal (CM) cells. They found that individual CM cells project monosynaptically to more than one motor nucleus and sometimes to muscles controlling different joints. Thus, muscles are not mapped one-to-one in cortical output neurons. Most of the neurons recorded by Fetz have phasictonic patterns of activity, firing most briskly during the dynamic phase of movement and settling down to a lower tonic rate when a steady force is reached (Figure 38-12A). For almost all neurons there is a range over which force is related linearly to firing rate. Often, however, this range is quite small, and maximal firing is achieved for relatively small forces Box 38-1 Postspike Facilitation of Muscle Activity Recording from cortical neurons in awake animals and relating the neuronal activity to movement parameters has led to significant insights about cortical control of movement. However, studies of this type are limited by their inability to identify functional connections between cortical neurons and the motor neurons of target muscles. This becomes possible with a technique developed by Ebehard Fetz and his colleagues called spike-triggered averaging (STA). Cortical motor neurons with direct excitatory synaptic connections to motor neurons produce individual EPSPs with a fixed latency. Any one EPSP is unlikely to fully depolarize a motor neuron but it transiently increases the probability the motor neuron will fire by bringing it closer to threshold. The EMG profile is the sum of spike trains of a population of motor units within a muscle and is a reliable indicator of the firing of spinal motor neurons. By averaging the EMG profile over thousands of discharges of one cortical neuron, the effect of a single cortical neuron on an EMG profile can be ascertained. This averaging subtracts out random associations of cortical neuronal firing and motor unit discharge; the signal-to-noise ratio improves with the number of discharges used to compile the average. Figure 38-11 shows the relation of the discharge of a single cortical neuron to an extension movement of the wrist. A cumulative average over 2000 discharges of the cortical neuron reveals a clear peak in the EMG profile beginning at a latency of 6 ms. This transient increase is called postspike facilitation and its short latency is interpreted as evidence of an underlying synaptic connection between the cortical neuron and the motor neurons.
- Figure 38-12 There is a direct relationship between the firing rate of motor cortical cells and force generation. (From Fetz EE and Cheney 1980.) A. Two types of motor cortical neurons, phasic-tonic and tonic, are predominant in the primary motor cortex. Each has a characteristic response pattern during isometric wrist torques in which the torque level is reached and held. (Similar patterns are seen for torques accompanied by wrist displacement.) 1. Phasic-tonic cell activity begins with a dynamic burst during the initial increase in torque and then decreases to a steady level when torque is maintained. 2. Tonic cell activity follows the rise in torque and remains at a high level. B. In both cell types activity increases with torque. The plot shows the relation between tonic firing rate, (impulses per second) and static torque during wrist extension.
- Figure 38-16 Whether an individual corticomotoneuronal (CM) cell is active depends on the motor task. The activity of a CM cell and the activity in its target muscle are not directly related. Cumulative histograms show the activity of a single neuron during a precision grip and a power grip. During the precision grip the neuron's activity is the same whether overall force is light or heavy and the level of electromyographic (EMG) activity in the target muscle is similar for both forces. During the power grip there is almost no activity in the neuron despite a greater amount of EMG activity in the muscle. Thus, even if a given motor neuron is monosynaptically connected to a given CM cell, their firing patterns do not have to parallel each other because the multiplicity of connections to motor neurons allows task flexibility. (imp/s = impulses per second.) (Maier et al 1993.) Roger Lemon and R. B. Muir demonstrated the importance of the task in determining which neurons in the primary motor cortex will be used to control a particular muscle. They examined the activity of individual CM cells in monkeys during two different finger tasks, a power grip and a precision grip, both of which involve contraction of the intrinsic hand muscles controlled by the identified CM cells. Cells that are active during the precision grip remain silent during the power grip, even though the contraction of the target muscle is stronger for the power grip than for the precision grip (Figure 38-16). The observation that activity in a CM cell is not invariably coupled with activation of its target muscle fundamentally distinguishes CM cells from spinal motor neurons. The finding that a distinct population of cells in the primary motor cortex is active only during the precision grip is further evidence of the special role of the primary motor cortex in controlling individuated movements of the fingers. The power grip, which does not require individual finger movements, can be controlled by descending pathways, arising either within or outside of the primary motor cortex, that diverge extensively in the spinal cord and therefore can recruit a large number of muscles in a less differentiated synergy. Certain motor cortical cells fire less and less often as muscle force increases. That is, their activity is correlated negatively with force. However, like neurons with positive correlations (see Figure 38-12), these cells also facilitate their target muscles. They discharge only during tasks that require precise control of force and smooth changes in force. Thus their function may be to provide more precise derecruiting of motor units than would be afforded simply by inhibiting the so-called positive cortical neurons. This would be helpful, for example, in releasing delicate objects carefully. In conclusion, the primary cortex has two levels of functional organization. First, a low-level control system, the CM cells, controls groups of muscles that can be brought together into task-specific combinations. Second, a higher-level control system encodes more global features of the movement. Practice and learning adjust the relation between these two levels of organization.
- Figure 38-17 Different areas of cortex are activated during simple, complex, and imagined sequences of finger movements. Local increases in cerebral blood flow during a behavior indicate which areas of motor cortex are involved in the behavior. In the experiment illustrated here blood flow was measured by intravenously injecting radioactive xenon dissolved in a saline solution and measuring the radioactivity over different parts of cortex using arrays of detectors placed over the scalp. Because local tissue perfusion varies with neural activity, the measured radioactivity provides a good index of regional activity in the surface of the brain. (Adapted from Roland et al. 1980.) A. When a finger is pressed repeatedly against a spring, increased blood flow is detected in the hand-control areas of the primary motor and sensory cortices. The increase in the motor area is related to the execution of the response, whereas the increase in the sensory area reflects the activation of peripheral receptors. B. During a complex sequence of finger movements the increase in blood flow extends to the medial premotor area, which includes the supplementary motor area (SMA) and presupplementary motor area (preSMA). C. During mental rehearsal of the same sequence illustrated in part B, blood flow increases only in the medial motor area. Each Premotor Area Contributes to Different Aspects of Motor Planning Although the outputs of the premotor areas and the primary motor cortex overlap in the spinal cord, the inputs to the premotor areas are quite different from those to the primary motor cortex (see Figure 38-4). Moreover, damage to premotor areas causes more complex motor impairments than does damage to primary motor cortex. When a monkey with a large lesion of the premotor area is presented with food behind a transparent shield it will reach directly for the food and bump into the shield. Unlike a normal animal it is unable to incorporate visuospatial information about the shield into the kinematic plan for moving its hand. The idea that premotor areas are involved in planning movement has received crucial support during the past 20 years from physiological and imaging studies of humans and monkeys performing a variety of special tasks. In monkeys distinct populations of cells are active in connection with ipsilateral movements, bilateral movements, or specific combinations of movements. Set-related and preparatory activity predominates, and cell activity is often associated primarily with specific tasks as we will see below. Studies of the premotor areas have identified several basic features of the neural organization of motor preparation. First, movements that are initiated internally by the subject—such as the sequencing of finger movements when manipulating an object—involve primarily the supplementary motor area. Second, movements triggered by external sensory events involve primarily the lateral premotor areas. More specifically, separate populations of lateral premotor neurons map the often arbitrary relationship between stimulus and response. The lateral dorsal premotor area is also concerned with delayed action (executed later on cue), whereas the lateral ventral premotor area is concerned with conforming the hand to the shape of objects. Third, mental rehearsal of a movement—that is, the use of visual imagery to plan a movement—invokes the same patterns of activity in the premotor and posterior parietal cortical areas as those that occur during performance of the movement. Psychophysical studies have shown that mental rehearsal of movement has a similar time course and closely simulates task performance. This observation helps explain the importance of mental rehearsal to athletes and skilled performers. Fourth, the premotor areas activated during a particular task are not the same over time but change progressively as performance becomes automatic. The Supplementary and Presupplementary Motor Areas Play an Important Role in Learning Sequences of Discrete Movements Motor actions are often self-initiated without an environmental cue. Nearly a full second before a self-initiated voluntary movement begins, a characteristic negative shift in cortical potentials is seen in the electroencephalogram (EEG) record of medial premotor regions, where the supplementary motor area is situated. This negative potential, referred to as the preparatory potential or Bereitschaft potential, signals the planning that occurs before movement is executed. The region responsible for this negative potential was localized more precisely in a study comparing increases in regional cerebral blood flow (a measure of increases in neuronal activity) during simple, complex, P.772 and imagined sequences of finger movements. Complex movement sequences require more planning than do simple repetitive movements. Imagining complex movements might require the same amount of planning as real movements. As expected, during forceful repetitive finger flexions against a spring-loaded movable cylinder, increases in regional cerebral blood flow were largely confined to the contralateral primary sensorimotor hand-control region. A complex sequence of finger movements was accompanied by regional cerebral blood flow increases within the supplementary motor area. Remarkably, when the complex sequence of finger movements was simply imagined, regional cerebral blood flow increased in an area anterior to the supplementary motor area on both sides (Figure 38-17). This area, the presupplementary motor area, provides the main input to the supplementary motor area and is discussed in detail below. The specific role of the supplementary motor area in the internal representation of sequences of movements was examined in another experiment, in which recordings were made from neurons in the primary motor cortex, supplementary motor area, and lateral premotor areas of monkeys while the animals performed two variations of an instructed-delay task. In this type of task subjects are taught which movements to make and later given a cue telling them when to make the movements. The monkeys in this experiment were instructed to touch three panels in a specific sequence. In one variation the instruction was visual: Three panels were lit up in a sequence that the monkeys had to follow. In the other variation the monkeys were instructed to perform a previously memorized sequence. As expected, neurons in the primary motor cortex generally discharged before and during movements to the same degree for visually guided and memorized sequences. In contrast, many supplementary motor area neurons fired only before and during performance of a memorized sequence. The reverse was true for the lateral premotor neurons (Figure 38-18). In addition, the movement-related discharge of some supplementary motor area neurons is specific to a particular sequence of movements such as pushing followed by turning a handle. The cells do not fire in connection with other combinations of the same movements. Thus the supplementary motor area seems to be involved in preparing movement sequences from memory in the absence of visual cues. The main cortical input to the supplementary motor area arises from the presupplementary motor area (see Figure 38-4). This region projects only to the supplementary motor area and has no clear somatotopy. Whereas the supplementary motor area is involved in setting the motor programs for learned sequences, the presupplementary motor area is thought to be involved in learning these sequences. For example, in one study the presupplementary motor area was preferentially activated while subjects learned a new sequence of button presses; the supplementary motor area became active only during the performance of the movements once they were learned. This motor learning likely involves a continuous interchange of information with the prefrontal cortex (area 46) and other areas of cortex. When proficiency and skill are gained, the neural control of task performance can also shift from the supplementary motor area to the primary motor cortex. In one recent study with monkeys, premovement activity in the supplementary motor area during the performance of a key-pressing task disappeared after 12 months of overtraining. Subsequently, an experimental lesion in the right primary motor cortex of these overtrained monkeys caused weakness in the left digits, thereby greatly compromising the monkeys' ability to perform the task. After 21 days the monkeys had recovered sufficiently to press the keys with the same skill as before they received the lesion. Twenty-two days after the monkeys received the lesion recordings from the supplementary motor area showed that neurons were again very active before movement. Much as extended practice influences the extent of P.774 motor representation in the primary motor cortex, a shift in representation occurs in the supplementary motor cortex as a task goes from being novel to automatic. Conversely, recovery of function following damage to the primary motor cortex represents a new learning challenge in which the supplementary and perhaps presupplementary motor areas participate anew.
- Figure 38-18 Cell activity in the motor cortex depends on whether a sequence of movements is guided by visual cues or by prior training. Monkeys were required to press three buttons either in a sequence presented by lighting three panels in turn or in a sequence they had learned previously. After being instructed to perform the observed sequence or the trained sequence, there was a delay before the animal was given a signal to initiate the movement. Raster plots represent cell discharge before and during movement on 16 trials, and the histogram shows the summed activity over all trials. Data are aligned to the onset of the first key touch. The cell in the primary cortex fired whether the sequence performed was the one learned in prior training or the one cued by lighted panels. The cell in the lateral premotor area fired only when the visually cued sequence was used, whereas the cell in the supplementary motor area fired only when the trained sequence was used. (From Mushiake 1991.)
- Figure 38-19 A set-related neuron in the dorsal premotor area becomes active while the monkey prepares to make a movement to the left. An instruction signal (illumination of one of four panels) tells the monkey which panel it will have to depress when a trigger signal (illumination of a nearby light-emitting diode) is presented. In the raster plots each dot on each line represents a spike in the recorded neuron. Each line is one trial, and successive trials are aligned on the onset of the instruction signal. The delay between the instruction and trigger signals varied randomly among three values. In the raster plots and histograms the responses made with each delay time are grouped to show that the discharge of the neuron coincides with the instruction signal and lasts until the response is made after the trigger signal. (From Weinrich and Wise 1982.) The Lateral Premotor Areas Contribute to the Selection of Action and to Sensorimotor Transformations Selection of appropriate action can be the result of internal reflection, which may involve evocation of mental imagery. More often, however, actions are responses to visual or auditory cues. Such cues may signify that a particular action is required immediately (eg, a red light telling us to stop) or that some type of situation is imminent in which action will be required (eg, a yellow light signaling an imminent change to red). The ability to learn new, adaptive responses to particular environmental stimuli is crucial to effective and accurate movement. We have seen that set-related activity occurs in the primary motor cortex and supplementary motor area before movement is executed. In the primary motor cortex this activity represents specific parameters of a particular movement; in the supplementary motor area it represents a specific order of responses. In the lateral premotor areas it represents how visual or other sensory stimuli are to be used to direct the movement. Characteristically, set-related activity in the premotor area persists during the entire interval between an anticipatory cue and the signal to move (Figure 38-19). Set-related activity in the lateral dorsal premotor area is related predominantly to sensory stimuli that do not convey spatial cues to direct movement. For example, the stimulus could be a light in a location that is not related to the direction in which the movement is to be executed. Thus the lateral dorsal premotor area is involved in learning to associate a particular sensory event with a specific movement (associative learning). Consistent with this, monkeys with lesions in the lateral dorsal premotor area have difficulty with associative learning. In one study monkeys were taught to associate pulling or pushing a joystick with a particular background light (red or blue). The lateral premotor cortex was then removed from both hemispheres and the animals were retrained two weeks after surgery. Although the monkeys were able to execute the required movements without impairment, none was able to relearn the association between the background color and whether to push or pull.
- Figure 38-20 The visuomotor transformations required for reaching and grasping involve two different pathways from the primary visual cortex to the premotor areas. Reaching. A path connects the parieto-occipital extrastriate area (PO) and the dorsal premotor area (PMd). Some of these connections reach PMd directly, and some relay via areas in the intraparietal sulcus: the medial dorsal parietal (MDP) and medial intraparietal (MIP) areas. This system is responsible for transforming visual information about the location of objects in extrapersonal space into the direction of a reaching movement. Grasping. A path connects the dorsal extrastriate (ES) cortex and the ventral premotor area (PMv) via the anterior intraparietal area (AIP). This system is responsible for transforming visual information about the properties of objects, such as shape and size, into commands for effective grasping. Reaching and Grasping Are Mediated by Separate Parieto-Premotor Channels Goal-directed movements require transformation of sensory representations of the environment into muscle-control signals, a process termed sensorimotor transformation. Reaching, a goal-directed movement, requires that visual information about target location and the position of the upper limb be used to specify critical features of the upcoming arm movement. In addition, reaching is commonly coupled with grasping an object. The parameters for reaching movement, notably direction and extent, depend on the location of the target relative to the body, shoulder, or hand. Grasping, in contrast, is governed mainly by the shape and dimensions of the object. Grasping involves first a separation of the fingers sufficient to enclose the object and then closure as the object is gripped between the pads. Separation of the fingers occurs during transport of the hand toward the object. The kinematics of grasping thus depend on the object itself and not on its location. Thus reaching and grasping are interesting behaviors to study in order to better understand the process of visuomotor transformation. Anatomical evidence and single-cell recordings have shown that separate but parallel parieto-premotor channels mediate visuomotor transformations required for reaching and grasping (Figure 38-20). During reaching, neurons in parietal area 5 code for direction of the movement but discharge later than dorsal premotor neurons to which they are connected. These neurons could monitor ongoing movements and improve the planning and execution of subsequent reaches by premotor areas.
- Figure 38-21 Individual neurons in the ventral premotor area fire during specific hand actions only. Raster plots and cumulative histograms show the discharge of a single neuron in the lateral ventral premotor area (F5) of a monkey during a precision grip and a power grip involving all the fingers. The cell is active during the precision grip by either arm but not during the power grip by either arm. Thus its activity is specific to the grip type employed by either hand. The fact that the neuron is active during movement of both arms excludes the possibility that this difference is due solely to the different patterns of corticospinal activation required by the two grips; if this were the case, only contralateral activation would occur. (From Rizzolatti et al. 1996.) During grasping, different neurons in the lateral ventral premotor area of monkeys fire in connection with different hand actions and object shapes. These neurons are active throughout reach, well before the fingers begin to grasp. Moreover, different cells fire during different patterns of hand shaping. Some neurons are active only when the action is a precision grip; others are active only when the action is a swiping movement to retrieve food; still others are active only if the action is a power grip (Figure 38-21A). The cells in the lateral ventral premotor area thus seem to direct motor acts that can be guided by visual information about object shape received from the posterior parietal cortex. Another set of neurons discharges whether an object is grasped or bitten
- Figure 38-22 An individual cell in the ventral premotor area is active whether the monkey performs a task or observes someone else perform the task. The fact that the same cell is active during action or observation suggests that it is involved in the abstract representation of the motor task. A. Activity in the neuron as the monkey observes another monkey make a precision group. B. Activity in the same neuron as the monkey observes the human experimenter make the precision grip. C. Activity in the same neuron as the monkey itself performs a precision grip. (From Rizzolotti et al 1996.) A cytoarchitectonic map of the monkey cortex and an example of a mirror neuron. The upper part of the figure shows the activity of a mirror neuron recorded from area F5. The neuron discharges both when the monkey grasps an object (A) and when it observes the experimenter grasping the object (B). (C) The cytoarchitectonic parcellation of the agranular frontal cortex and the parietal lobe. PE, PEc, PEip, PF, PFG and PG are parietal areas. An enlargement of the frontal region (inset on the left) shows the parcellation of area F5 into three parts: F5c, F5p and F5a. The mirror neurons are typically found in F5c. The inset on the right shows the areas buried within the intraparietal sulcus. Abbreviations: Al, inferior arcuate sulcus; AlP, anterior intraparietal area; AS, superior arcuate sulcus: C, central sulcus: FEF, frontal eye field; IO, inferior occipital sulcus: IP, inferior precentral sulcus; L, lateral sulcus: LIP, lateral intraparietal area; Lu, lunate sulcus: MIP, medial intraparietal area; P, principal sulcus; STS, superior temporal sulcus: VIP, ventral intrapanetal area. Permission obtained from Elsevier Ltd © Rizzolatti G and FabbriD Destoo M (2008) Cuff Opin Neurobiol 18: 179—184. Mirror Neurons in the Monkey Mirror neurons were originally discovered in the ventral premotor cortex (area F5) of the macaque monkey.8’9i The defining charactenstic ot these neurons is that they discharge both when the monkey performs a motor act and when the monkey, at rest, observes another individual (a human being or another monkey) performing a similar motor act (Figure 1). The degree of similarity that is required between executed and observed motor acts in order to trigger a given mirror neuron varies from one neuron to another. For most mirror neurons, however, the relationship between the effective observed and executed motor acts is based on their common goal (e.g. grasping), regardless of how this goal is achieved (e.g. using a twof iinger or a whole-hand prehension). Importantly, mirror neurons do not discharge in response to the presentation of food or other interesting objects. Mirror neurons have also been described in the PFG and anterior intraparietal areas of the inferior parietal lobule (IPL: Figure 1). The general properties of parietal mirror neurons seem to be similar to those of mirror neurons in the premotor cortex. Like the latter neurons, the panetal mirror neurons code for the goals of motor acts rather than the movements from which they are con stncted [8.9J The PFG and anterior intraparetal areas are both connected with the F5 area and the cortex of the superior temporal sulcus. Neurons in the superior temporial sulcus have complex visual properties, and some respond to the observation of motor acts done by others.i01i1 However, they lack the motor properties that are defining features of mirror neurons, and cannot, therefore, be considered to be part of the mirror system. Mirror neurons represent a distinctive class of neurons that discharge both when the monkey executes a motor act and when it observes another individual (a human being or another monkey) performing the same or a similar motor act (Figure 1 ). These neurons do not discharge in response to the simple presentation of food or of other interesting objects. They also do not discharge, when the monkey observes hand actions mimicked without the target object. Thus, the effective visual stimulus is the observation of a hand interacting with an object (Gallese et al. 1996, Rizzolatti et al. 1996a). Originally discovered in a subdivision of the monkey's premotor cortex , area F5, mirror neurons have later been also found in the inferior parietal lobule (IPL, Rizzolatti et al. 2001, Fogassi et al. 2005) (Figure 2 ). IPL receives a strong input from the cortex of the superior temporal sulcus (STS), a region known to code biological motion (Jellema et al. 2002), and sends output to ventral premotor cortex including area F5. Note that, although STS responds to the observation of actions done by others, it is not endowed with motor properties. Thus, the cortical mirror neuron system is formed by two main regions: the ventral premotor cortex and the rostral part of the inferior parietal lobule. Neurophysiological ( EEG , MEG , and TMS ), and brain-imaging ( PET and fMRI ) experiments provided strong evidence that a fronto-parietal circuit with properties similar to the monkey's mirror neuron system is also present in humans (Rizzolatti and Craighero 2004). As in the monkey the mirror neuron system is constituted of IPL and a frontal lobe sector formed by the ventral premotor cortex plus the posterior part of the inferior frontal gyrus (IFG) (Figure 3 ). A unique type of neuron has been discovered in the lateral ventral premotor area. Like others, these neurons discharge when the monkey performs a specific grasping movement, but they also discharge when the monkey observes the same movement being made by another monkey or even by the experimenter. These neurons have been called mirror neurons (Figure 38-22). These differnt neurons all share the characteristic of encoding a vocabulary of goal-directed behaviors rather than how these behaviors will be carried out. The vental premotor area receives its main input from neurons with similar task related properties in the anterior intraparietal region, a region buried in the intraparietal sulcus. Recordings of these neurons were made while a monkey performed a series of tasks involving several different switches and knobs. Cells fired selectively when particular switches were grasped and also fired when the monkey visually fixated the same switch without grasping it. These cells may have a role in transforming the dimensions of an object in visual space into motor signals. Reaching and Grasping Are Mediated by Separate Parieto-Premotor Channels Goal-directed movements require transformation of sensory representations of the environment into muscle-control signals, a process termed sensorimotor transformation. Reaching, a goal-directed movement, requires that visual information about target location and the position of the upper limb be used to specify critical features of the upcoming arm movement. In addition, reaching is commonly coupled with grasping an object. The parameters for reaching movement, notably direction and extent, depend on the location of the target relative to the body, shoulder, or hand. Grasping, in contrast, is governed mainly by the shape and dimensions of the object. Grasping involves first a separation of the fingers sufficient to enclose the object and then closure as the object is gripped between the pads. Separation of the fingers occurs during transport of the hand toward the object. The kinematics of grasping thus depend on the object itself and not on its location. Thus reaching and grasping are interesting behaviors to study in order to better understand the process of visuomotor transformation. Anatomical evidence and single-cell recordings have shown that separate but parallel parieto-premotor channels mediate visuomotor transformations required for reaching and grasping (Figure 38-20). During reaching, neurons in parietal area 5 code for direction of the movement but discharge later than dorsal premotor neurons to which they are connected. These neurons could monitor ongoing movements and improve the planning and execution of subsequent reaches by premotor areas. During grasping, different neurons in the lateral ventral premotor area of monkeys fire in connection with different hand actions and object shapes. These neurons are active throughout reach, well before the fingers begin to grasp. Moreover, different cells fire during different patterns of hand shaping. Some neurons are active only when the action is a precision grip; others are active only when the action is a swiping movement to retrieve food; still others are active only if the action is a power grip (Figure 38-21A). The cells in the lateral ventral premotor area thus seem to direct motor acts that can be guided by visual information about object shape received from the posterior parietal cortex. Another set of neurons discharges whether an object is grasped or bitten. A unique type of neuron has been discovered in the lateral ventral premotor area. Like others, these neurons discharge when the monkey performs a specific grasping movement, but they also discharge when the monkey observes the same movement being made by another monkey or even by the experimenter. These neurons have been called mirror neurons (Figure 38-22). These differnt neurons all share the characteristic of encoding a vocabulary of goal-directed behaviors rather than how these behaviors will be carried out. The vental premotor area receives its main input from neurons with similar task related properties in the anterior intraparietal region, a region buried in the intraparietal sulcus. Recordings of these neurons were made while a monkey performed a series of tasks involving several different switches and knobs. Cells fired selectively when particular switches were grasped and also fired when the monkey visually fixated the same switch without grasping it. These cells may have a role in transforming the dimensions of an object in visual space into motor signals. Sensory Motor Talents and Cortical Space Are special sensory motor talents, such as the exceptional speed and coordination displayed by talented athletes, ballet dancers, or concert musicians, visible in the structure of the nervous system? The widespread use of noninvasive brain imaging techniques (see Boxes B and C in Chapter 1) has generated a spate of studies that have tried to answer this and related questions. Most of these studies have sought to link particular sensory motor skills to the amount of brain space devoted to such talents. For example, a study of professional violinists, cellists, and classical guitarists purported to show that representations of the “fingering” digits of the left hand in the right primary somatic sensory cortex are larger than the corresponding representations in nonmusicians. Although such studies in humans remain controversial (the techniques are only semiquantitative), the idea that greater motor talents (or any other ability) will be reflected in a greater amount of brain space devoted to that task makes good sense. In particular, comparisons across species show that special talents are invariably based on commensurately sophisticated brain circuitry, which means more neurons, more synaptic contacts between neurons, and more supporting glial cells—all of which occupy more space within the brain. The size and proportion of bodily representations in the primary somatic sensory and motor cortices of various animals reflects species-specific nuances of mechanosensory discrimination and motor control. Thus, the representations of the paws are disproportionately large in the sensorimotor cortex of raccoons; rats and mice devote a great deal of cortical space to representations of their prominent facial whiskers; and a large fraction of the sensorimotor cortex of the star-nosed mole is given over to representing the elaborate nasal appendages that provide critical mechanosensory information for this burrowing species. The link between behavioral competence and the allocation of space is equally apparent in animals in which a particular ability has diminished, or has never developed fully, during the course of evolution. Nevertheless, it remains uncertain how—or if—this principle applies to variations in behavior among members of the same species, including humans. For example, there does not appear to be any average hemisphere asymmetry in the allocation of space in either the primary sensory or motor area, as measured cytoarchitectonically. Some asymmetry might be expected simply because 90% of humans prefer to use the right hand when they perform challenging manual tasks. It seems likely that individual sensory motor talents among humans will be reflected in the allocation of an appreciably different amount of space to those behaviors, but this issue is just beginning to be explored with quantitative methods that are adequate to the challenge. The Premotor Cortex A complex mosaic of interconnected frontal lobe areas that lie rostral to the primary motor cortex also contributes importantly to motor functions (see Figure 17.7). The upper motor neurons in this premotor cortex influence motor behavior both through extensive reciprocal connections with the primary motorcortex, and directly via axons that project through the corticobulbar and corticospinal pathways to influence local circuit and lower motor neurons of the brainstem and spinal cord. Indeed, over 30% of the axons in the corticospinal tract arise from neurons in the premotor cortex. In general, a variety of experiments indicate that the premotor cortex uses information from other cortical regions to select movements appropriate to the context of the action (seeChapter 26). The functions of the premotor cortex are usually considered in terms of the lateral and medial components of this region. As many as 65% of the neurons in the lateral premotor cortex have responses that are linked in time to the occurrence of movements; as in the primary motor area, many of these cells fire most strongly in association with movements made in a specific direction. However, these neurons are especially important in conditional motor tasks. Thus, in contrast to the neurons in the primary motor area, when a monkey is trained to reach in different directions in response to a visual cue, the appropriately tuned lateral premotor neurons begin to fire at the appearance of the cue, well before the monkey receives a signal to actually make the movement. As the animal learns to associate a new visual cue with the movement, appropriately tuned neurons begin to increase their rate of discharge in the interval between the cue and the onset of the signal to perform the movement. Rather than directly commanding the initiation of a movement, these neurons appear to encode the monkey's intention to perform a particular movement; thus, they seem to be particularly involved in the selection of movements based on external events. Further evidence that the lateral premotor area is concerned with movement selection comes from studies of the effects of cortical damage on motor behavior. Lesions in this region severely impair the ability of monkeys to perform visually cued conditional tasks, even though they can still respond to the visual stimulus and can perform the same movement in a different setting. Similarly, patients with frontal lobe damage have difficulty learning to select a particular movement to be performed in response to a visual cue, even though they understand the instructions and can perform the movements. Individuals with lesions in the premotor cortex may also have difficulty performing movements in response to verbal commands. The medial premotor cortex, like the lateral area, mediates the selection of movements. However, this region appears to be specialized for initiating movements specified by internal rather than external cues. In contrast to lesions in the lateral premotor area, removal of the medial premotor area in a monkey reduces the number of self-initiated or “spontaneous” movements the animal makes, whereas the ability to execute movements in response to external cues remains largely intact. Imaging studies suggest that this cortical region in humans functions in much the same way. For example, PET scans show that the medial region of the premotor cortex is activated when the subjects perform motor sequences from memory (i.e., without relying on an external instruction). In accord with this evidence, single unit recordings in monkeys indicate that many neurons in the medial premotor cortex begin to discharge one or two seconds before the onset of a self-initiated movement. In summary, both the lateral and medial areas of the premotor cortex are intimately involved in selecting a specific movement or sequence of movements from the repertoire of possible movements. The function of the areas differs, however, in the relative contributions of external and internal cues to the selection process.
- Figure 2. Action-constrained neurons in the monkey IPL. (A) Apparatus and paradigm used for a task designed to demonstrate action-constrained neurons. The monkey starts from the same position in all trials, reaches for an object (1) and brings it to the mouth (2a) or places it into a container (2b). (B) Activity of three IPL neurons during the motor task in conditions 2a (grasp to place) and 2b (grasp to eat). Raster histograms are synchronized with the moment when the monkey touched the object to be grasped. Unit 67 fires during grasping to eat and not during grasping to place. Unit 161 is selective for grasping to place. Unit 158 does not show any task preference. (C) Visual responses of IPL mirror neurons during the observation of grasping to eat and grasping to place performed by an experimenter. Unit 87 is selective for grasping to eat, unit 39 is selective for grasping to place and unit 80 does not display any task preference. Abbreviation: IPL, inferior parietal lobule. Permission obtained from American Association for the Advancement of Science © Fogassi L et a!. (2005) Science 308: 662-667. Action understanding and imitation The original hypothesis on the functional role of mirror neuron system was that of action understanding (Rizzolatti et al. 2001). It may sound bizarre that in order to recognize an action, one should activate the motor system . However, as a matter of fact this is not so strange. A mere visual perception , without involvement of the motor system would only provide a description of the visible aspects of the movements. It would not give, however, information on the intrinsic components of the observed action, on what it means to do the action, and on the links between the observed action and other actions related to it. This can be achieved only if the observed action is mapped onto the motor system of the observer. Thus, the activation of the mirror circuit is essential to provide the observer with a real experiential comprehension of the observed action. This comprehension links the observer of the action with its agent, creating a rudimentary form of social interactions. On top of this function, other functions can be built, some of which are present only in humans. From this perspective, mirror neurons could represent a "core mechanism" from which other functions branched off. One of these is imitation, that is the ability to replicate an observed action already present in the observer motor repertoire or to learn a new motor action. The mirror neuron system, by providing motor copies of the observed actions, appears to be the ideal mechanism for imitation. Indeed there is clear evidence that mirror neuron system is involved both in immediate repetition of actions done by others (Iacoboni et al. 1999) and in imitation learning (Buccino et al. 2004). While immediate repetition of an observed action is carried out by the mirror system itself, imitation learning requires the intervention of the prefrontal lobe (Iacoboni et al. 1999). This lobe (area 46 in particular) combines elementary motor acts into more complex motor patterns. [ edit ] Intention understanding There are two distinct series of information that one can get observing an action done by another individual. One is "what" the actor is doing; the other is "why" the actor is doing it. If we see, for example, a girl grasping an apple, we understand that she is grasping an object. Often, we can also understand, in addition, why she is doing it, that is we can understand her intention. We can infer if she is grasping the apple for eating it, or for putting it into a basket. Although the hypothesis that mirror neurons are involved in intention understanding has been proposed several years ago (Gallese and Goldman 1998), only recently, however, this hypothesis has been supported by an fMRI experiment. In this experiment volunteers were presented with hand actions without a context and hand actions executed in contexts that allowed them to understand the intention of the action agent. The main result of the study was the demonstration that actions embedded in contexts yielded selective activation of the mirror neuron system. This indicates that mirror areas, in addition to action understanding, also mediate the understanding of others’ intention (Iacoboni et al. 2005). These data indicate that the mirror neuron system is involved in intention understanding, without providing, however, information on the specific mechanisms underlying it. In order to elucidate these mechanisms monkeys were trained to perform two actions with different goals (Fogassi et al. 2005). In the first, the monkey had to grasp an object in order to place it into container, in the second it had to grasp a piece of food to eat it. The initial motor acts, reaching and grasping, were identical in the two conditions, while the final goal of the two actions was different. The activity of single neurons was recorded from the inferior parietal lobule (IPL). The results showed that many of IPL neurons discharge selectively when the monkey executes a given motor act (e.g. grasping). Very interestingly, most of them fire only when the coded motor act is followed by a subsequent specific motor act (e.g. placing). Some of these "action-constrained" motor neurons had mirror properties and selectively discharged during the observation of motor acts when these were embedded in a given action (e.g., grasping-for-eating but not grasping-for-placing). Thus, the activation of IPL action-constrained mirror neurons give information not only about, but also on why grasping is done (grasping-for-eating or grasping-for placing). This specificity allowed the observer not only to recognize the observed motor act, but also to code what will be the next motor act of the not-yet-observed action: In other words to understand the intentions of the action’s agent. It has been suggested that there is a link between autism and mirror neuron system. According to this view, the inability of autistic children to relate to people and life situations in the ordinary way depends on a lack of a normally functioning mirror neuron system. Recent neurophysiological and brain imaging studies provided evidence in favor of this hypothesis (Ramachandran and Oberman 2006; Dapretto et al. 2006). It is generally assumed that the primary deficit in intention understanding found in autistic children, is due to damage of the mirror system as the system responsible for understanding the actions of others. However, one may wonder whether the primary deficit in autism lies indeed in the incapacity to understand others or rather in a more basic deficits in the organization of the motor chains. In other words, the fundamental deficit in autistic children resides in the incapacity to organize their own intentional motor behavior.
- Umiltà et al. studied the responses of F5 mirror neurons in two conditions. In the first one, the monkey could see the whole action made by the experimenter (full-vision condition). In the second, the monkey could see only the beginning of the same action; the crucial part - the hand/object interaction - was hidden from view (hidden condition), although the monkey was shown that an object or some food had previously been located behind the screen. So, the meaning of the experimenter's action could be inferred from the monkey's knowledge of the situation and the view of the hand disappearing behind the screen. The results showed that more than half of the recorded mirror neurons also discharged in the hidden condition. This indicates that, despite the fact that the monkey did not see the action, it knew its meaning; its neurons signalled 'the experimenter is grasping' or 'the experimenter is holding' . This argues against the need for a visual description of action for action understanding, and therefore oppose the visual hypothesis.
- MIRRORING INTENTIONS The following graphic, taken from Rizzolatti et al.'s article in the Nov. 2006 Scientific American, shows that firing of neurons in the inferior parietal lobe of the macaque monkey can discriminate intention, noting the difference between placing a food object in the mouth or in a bowl.
- Schematic overview of the frontoparietal mirror neuron system (MNS) (red) and its main visual input (yellow) in the human brain. An anterior area with mirror neuron properties is located in the inferior frontal cortex, encompassing the posterior inferior frontal gyrus (IFG) and adjacent ventral premotor cortex (PMC) 33, 36, 38, 45, 46, 48, 53, 62 . A posterior area with mirror neuron properties is located in the rostral part of the inferior parietal lobule (IPL), and can be considered the human homologue of area PF/PFG in the macaque 55, 109 . The main visual input to the MNS originates from the posterior sector of the superior temporal sulcus (STS) 113 . Together, these three areas form a 'core circuit' for imitation 41 . The visual input from the STS to the MNS is represented by an orange arrow. The red arrow represents the information flow from the parietal MNS, which is mostly concerned with the motoric description of the action 33, 40 , to the frontal MNS, which is more concerned with the goal of the action 33, 38 . The black arrows represent efference copies of motor imitative commands that are sent back to the STS to allow matching between the sensory predictions of imitative motor plans and the visual description of the observed action 42 . Anatomical image adapted, with permission, from Ref. 114 © (1996) Appleton & Lange.
- Figure 3. The parietofrontal mirror system in humans. Lateral view of the human cerebral cortex showing Brodmann cytoarchitectonic subdivision. The areas in yellow correspond to areas that respond to the observation and execution of hand motor acts. The left-hand panel shows an enlarged view of the frontal lobe. The possible homology between monkey and human premotor cortex is indicated by arrows. Note that in monkeys area F5 consists of three subareas: F5c, F5p and F5a. Area 44 is considered to be the most likely human homolog of area F5. Abbreviations: C, central sulcus; FEF, frontal eye field; IF, inferior frontal sulcus; IP, inferior precentral sulcus: PMd, dorsal premotor cortex; PMv, ventral premotor cortex; PrePMd, pre-dorsal premotor cortex; SF, supenor frontal sulcus; SP, upper part of the superior precentral sulcus. Permission obtained from Elsevier Ltd © Rizzolatti G and Fabbn-Destro M (2008) Cuff Opin Neurobiol 18: 179—184. The Mirror System In Humans Understanding of Goals and Intentions A large number of studies based on noninvasive electrophysiological (e.g. EEG, magnetoencephalography [MEG]) or brain imaging (e.g. PET, functional MRI [fMRI]) techniques have demonstrated the existence of the mirror mechanism in humans.1891 Brain imaging studies have enabled the mirror areas to be located. These studies showed that the observation of transitive actions done by others results in an increase in blood oxygen level-dependent (BOLD) signal not only in visual areas, but also in the IPL and the ventral premotor cortex, as well as the caudal part of the inferior frontal gyrus (iFG). These latter three areas have motor properties and closely correspond to the areas that contain mirror neurons in the monkey (Figure 3). Both the premotor and the parietal areas of the human mirror system show a somatotopic organization j241 Observation of motor acts done with the leg, hand or mouth activates the precentral gyns and the pars opercularis of the IFG in a medial-to-lateral direction, as in the classical homunculus model of Penfield’25 and woolseyi26i In the IPL, mouth motor acts are represented rostrally, hand and arm motor acts are represented caudally, and leg motor acts are represented even more caudally and dorsally, extending into the superior parietal lobule. Most studies on the mirror mechanism in humans have investigated transitive movements such as grasping. In a recent fMRl study in which volunteers were asked to observe video clips showing a hand transport movement without an effector-object interaction, activations were found in the dorsal premotor cortex and also in the superior parietal lobule, with the adtivation extending into the intraparietal sulcus.271 This finding indicates that the human brain is endowed with a reaching mirror mechanism that is anatomically separated from the mirror mechanism that codes for the distal motor act. As in the monkey, the parietal and frontal mirror areas in humans code mostly for the goals of motor acts. Gazzola et aI.t28 instructed volunteers to observe either a human or a robot arm grasping objects. In spite of differences in shape and kinematics between the human and robot arms, the parietofrontal mirror network was activated in both conditions. Further evidence in favor ot goal coding was obtained in an fMRl study based on repetition suppression1o1—a technique that exploits the trial-by-tral reduction of a physiological response to repeated stimuli. The results showed that repeated presentation of the same goal caused suppression of the hemodynamic response in the left intraparietal sulcus, but this region was not sensitive to the trajectory of the agent’s hand. The study of aplasic individuals bomn without arms and hands provided further evidence in favor of a goal-coding mirror mechanism.1301 During MRI scanning, two aplasic individuals and a group of nonaplasic volunteers were instructed to watch videos showing hand actions. All participants also made adtions with their feet, mouths, and, in the case of the nonaplasic volunteers, hands. The results showed that in aplasic individuals, the observation of hand motor adts, which they had never themselves perfommed, adtivated the mirror areas. The communality of goals between the never-executed hand motor adts and those performed with the mouth and feet was the most probable explanation for this adtivation. Growing evidence exists that, in addition to goal coding, the human mirror mechanism has a role in the ability to understand the intentions behind the actions of others. In an fMRI study, volunteers observed motor acts (e.g. grasping a cup) embedded in specific contexts (a condition in which the agent’s intention could be easily understood) or devoid of context (a condition in which the agent's intention was ambiguous).131 The results showed that the mirror network was active in both conditions. However, the understanding of intention produced a stronger signal increase in the caudal IFG of the right hemisphere. The importance of the mirror system in understanding the intentions of others was confirmed by a repetition-suppression fMRI expenmenti321 Participants were asked to observe repeated movies showing either the same movement or the same action outcome regardless of the executed movement. The result showed activity suppression in the right IPL and the right IFG when the outcome was the same.
- Functional MRI (fMRI) study of imitation of finger movements showing two human cortical areas with the predicted pattern of activity for mirror neuron areas 33 . a | Participants observed or imitated the lifting of the index or the middle finger (top). In visual control conditions they observed a cross appearing on the index or middle finger of a static hand (middle), or appearing on the left or right side of a grey rectangle (bottom). In motor control conditions, partipants lifted the index or middle finger in response to the appearance of the cross. b | The two areas showing the predicted pattern of higher activity for the control motor task compared with action observation, and highest activity during imitation, were located in the inferior frontal cortex (Brodmann's area 44; BA44) and in the rostral part of the posterior parietal cortex (PPC) 33 . c | Blood-oxygen-level-dependent (BOLD) fMRI activity in signal intensity rescaled by smoothing measured in BA44 shows the predicted pattern of activity for mirror neuron areas. Panels a and b reproduced, with permission, from Ref. 33 © (1999) American Association for the Advancement of Science.
- The observation of a grasping action embedded in two different contexts ( a ) that suggest two different intentions — drinking on the left and cleaning up on the right — elicits differential activity (greater for drinking) in the mirror neuron area located in the right posterior inferior frontal gyrus 55 ( b ). This shows that the mirror neuron system does not simply code the observed action ('that's a grasp') but rather the intention associated with the action ('that's a grasp to drink'). Panel a modified from Ref. 55 .
- Marco Iacoboni et al. (PloS Biology, 3:1-7 (2005)) used functional magnetic resonance imaging in humans to investigate "Grasping the Intentions of Others with One's Own Mirror Neuron System" Twenty-three subjects watched three kinds of stimuli: grasping hand actions without a context, context only (scenes containing objects), and grasping hand actions performed in two different contexts. The images are organized in three columns and two rows. Each column corresponds to one of the experimental conditions. From left to right: Context, Action, and Intention. In the Context condition there were two types of clips, a "before tea" context (upper row) and an "after tea" context (lower row). In the Action condition two types of grips were displayed an equal number of times, a whole-hand prehension (upper row) and a precision grip (lower row). In the Intention condition there were two types of contexts surrounding a grasping action. The "before tea" context suggested the intention of drinking (upper row), and the "after tea" context suggested the intention of cleaning (lower row). Whole-hand prehension (displayed in the upper row of the Intention column) and precision grip (displayed in the lower row of the Intention column) were presented an equal number of times in the "drinking" Intention clip and the "cleaning" Intention clip. the Intention condition contained information that allowed the understanding of intention, whereas the Action and Context conditions did not (i.e., the Action condition was ambiguous, and the Context condition did not contain any action).
- As expected, given the complexity of the stimuli, large increases in neural activity were observed in occipital, posterior temporal, parietal, and frontal areas (especially robust in the premotor cortex) for observation of the Action and Intention conditions. Notably, the observation of the Intention and of the Action clips compared to rest yielded significant signal increase in the parieto-frontal cortical circuit for grasping. This circuit is known to be active during the observation, imitation, and execution of finger movements (''mirror neuron system'') Actions embedded in contexts, compared with the other two conditions, yielded a significant signal increase in the posterior part of the inferior frontal gyrus and the adjacent sector of the ventral premotor cortex where hand actions are represented.
- The subtractions high light differences in posterior part of the inferior frontal gyrus and the adjacent sector of the ventral premotor cortex where hand actions are represented. Upper row.....The Intention condition yielded significant signal increases-compared to the Action condition-in visual areas and in the right inferior frontal cortex, in the dorsal part of the pars opercularis of the inferior frontal gyrus Lower row ... in the Intention condition minus the Context condition signal increase was also found in the same right inferior frontal cortex previously seen activated in the comparison of the Intention condition versus Action condition. Thus, the differential activation in inferior frontal cortex observed in the Intention condition versus Action condition, cannot be simply due to the presence of objects in the Intention clips, given that the Context clips also contain objects. Thus, premotor mirror neuron areas-areas active during the execution and the observation of an action-previously thought to be involved only in action recognition are actually also involved in understanding the intentions of others. To ascribe an intention is to infer a forthcoming new goal, and this is an operation that the motor system does automatically. The conventional view on intention understanding is that the description of an action and the interpretation of the reason why that action is executed rely on largely different mechanisms. This works shows that the intentions behind the actions of others can be recognized by the motor system using a mirror mechanism. They suggest that coding the intention associated with the actions of others is based on the activation of a neuronal chain formed by mirror neurons coding the observed motor act and by "logically related" mirror neurons coding the motor acts that are most likely to follow the observed one, in a given context. To ascribe an intention is to infer a forthcoming new goal, and this is an operation that the motor system does automatically
- MIRROR SYSTEMS AND SELF AND OTHER I want to mention a clever experiment: distinction of self and other in mirroring motor neurons. The fact that the brain might represent others' actions like one's own raise the issue of how we distinguish self from other. What keeps us from constantly miming the actions of others? (This happens in echolalia, the involuntary repetition of words being heard that occurs in many persons with autism. ecopraxia, the involuntary mimicking of movements) Schütz-Bosbach et al (Current Biology 16:1830-1834 (2006)) have done a very clever experiment to examine this by manipulating the sense of body ownership (using the "rubber-hand illusion") to compare effects of observing actions that either were or were not illusorily attributed to the subject's own body. Let me give you their description of the experiment: When subjects watch a rubber hand being stroked while they feel synchronous stroking of their own unseen hand, they feel that the rubber hand becomes part of their body. Identical asynchronous stroking has no effect. Thus, the sense of owning the rubber hand requires congruence of visual and tactile stimulation. The neural counterparts of this sense of ownership have been identified in premotor and sensorimotor cortices. The rubber-hand illusion therefore allows balanced comparison between the self and the other because a single stimulus is either linked to the self or not depending on the pattern of previous stimulation. They used a real human hand instead of the conventional rubber hand because several studies show stronger mirroring effects for viewing a live action than for viewing artificial equivalents. They show that observing another's actions facilitated the motor system, like in the examples we've seen...... whereas observing identical actions, which were illusorily attributed to the subject's own body, showed the opposite pattern. Thus, motor facilitation strongly depends on the agent to whom the observed action is attributed. This result contradicts the idea of close equivalence between one's own actions and actions of others and suggests that social differentiation, not equivalence, is characteristic of the human action system.... Their quote: "This suggests that the neural mechanisms underlying action observation are intrinsically social. These mechanisms map the actions of others to corresponding actions on one's own body but do not simply represent the other agent as a derivative of, or even an equal to, the self." In contrast, there appear to be an agent-specific representation in the primary motor cortex." WHOA.... look how much is going on in what we previously thought to be simple old motor cortex that just plans and executes actions, its doing intentionality, self vs. others, etc. There aren't distinct modular or phrenological type intentionality, self vs. other brain areas, these functions get carried out hear the relevant sites of sensing and acting, just as memories are distributed over the cortex among areas relevant to their context, just as vision isn't just he visual cortex but happens in the 80+ percent of cortical neurons whose firing can be influenced by visual input.
