Japan medical device approval chart - Emergo
•
5 j'aime•4,560 vues
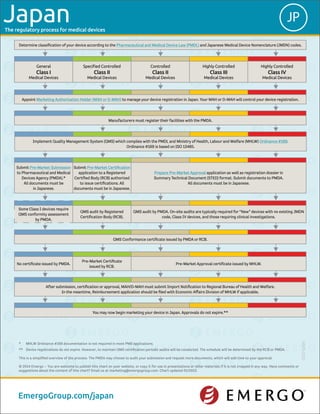
The regulatory process for medical devices in Japan involves several key steps: 1) Classifying the device and appointing a Marketing Authorization Holder who will manage the registration process. 2) Implementing a Quality Management System that complies with relevant regulations. 3) Submitting an application to either the Pharmaceuticals and Medical Devices Agency or a Registered Certification Body, depending on the device class. 4) Undergoing an audit and receiving the appropriate certificate before marketing the device in Japan. Device registrations do not expire but must be renewed periodically.
Signaler
Partager
Signaler
Partager
Télécharger pour lire hors ligne
Recommandé
Recommandé
Contenu connexe
Tendances
Tendances (20)
Australia medical device registration and approval process - EMERGO

Australia medical device registration and approval process - EMERGO
Japan PMDA Medical Device Regulatory Approval Process

Japan PMDA Medical Device Regulatory Approval Process
Regulatory approval process for invitro diagnostics in us

Regulatory approval process for invitro diagnostics in us
Medical device regulation US, European Union and India

Medical device regulation US, European Union and India
Market authorisation checklist for brics countries

Market authorisation checklist for brics countries
South Korea medical device approval chart - Emergo 

South Korea medical device approval chart - Emergo
En vedette
En vedette (9)
Digital & Social Marketing for Medical Devices

Digital & Social Marketing for Medical Devices
Choosing the right facility for clinical trials in Russia

Choosing the right facility for clinical trials in Russia
Similaire à Japan medical device approval chart - Emergo
Similaire à Japan medical device approval chart - Emergo (20)
Key Differences between Medical Device User Fees and Establishment Registrati...

Key Differences between Medical Device User Fees and Establishment Registrati...
Registration of medical devices with Brazil's Anvisa 

Registration of medical devices with Brazil's Anvisa
Overview of Japanese Foreign Manufacturers Accreditation

Overview of Japanese Foreign Manufacturers Accreditation
REGULATORY REQUIREMENTS FOR REGISTRATION OF DRUGS AND POST APPROVAL REQUIREME...

REGULATORY REQUIREMENTS FOR REGISTRATION OF DRUGS AND POST APPROVAL REQUIREME...
Strategies for Device Approval in China, India, South Korea and Australia

Strategies for Device Approval in China, India, South Korea and Australia
CE Marking , FDA Approval and Associated Regulations for Wellness

CE Marking , FDA Approval and Associated Regulations for Wellness
Dernier
❤️Chandigarh Escorts Service☎️9815457724☎️ Call Girl service in Chandigarh☎️ Chandigarh Call Girls Service ☎️ Call Girls In Chandigarh BEST Call Girls in CHANDIGARH Escort Service provide Cute Nice sweet and Sexy Models in beautiful CHANDIGARH city cash in hand to hand call girl in CHANDIGARH and CHANDIGARH escorts. HOT & SEXY MODELS // COLLEGE GIRLS IN CHANDIGARH AVAILABLE FOR COMPLETE ENJOYMENT WITH HIGH PROFILE INDIAN MODEL AVAILABLE HOTEL & HOME ★ SAFE AND SECURE HIGH CLASS SERVICE AFFORDABLE RATE ★ 100% SATISFACTION,UNLIMITED ENJOYMENT. ★ All Meetings are confidential and no information is provided to any one at any cost.
★ EXCLUSIVE Profiles Are Safe and Consensual with Most Limits Respected
★ Service Available In: - HOME & 24x7 :: 3 * 5 *7 *Star Hotel Service .In Call & Out call
Services :
★ A-Level (5 star escort)
★ Strip-tease
★ BBBJ (Bareback Blowjob)Receive advanced sexual techniques in different mode make their life more pleasurable.
★ Spending time in hotel rooms
★ BJ (Blowjob Without a Condom)
★ Completion (Oral to completion)
★ Covered (Covered blowjob Without a Condom)Chandigarh Call Girls❤️Chandigarh Escorts Service☎️9815457724☎️ Call Girl service in Chandigarh☎️ ...

❤️Chandigarh Escorts Service☎️9815457724☎️ Call Girl service in Chandigarh☎️ ...Rashmi Entertainment
❤️Chandigarh Escort Service☎️9815457724☎️ Call Girl service in Chandigarh☎️ Chandigarh Call Girls Service ☎️ Call Girls In Chandigarh BEST Call Girls in CHANDIGARH Escort Service provide Cute Nice sweet and Sexy Models in beautiful CHANDIGARH city cash in hand to hand call girl in CHANDIGARH and CHANDIGARH escorts. HOT & SEXY MODELS // COLLEGE GIRLS IN CHANDIGARH AVAILABLE FOR COMPLETE ENJOYMENT WITH HIGH PROFILE INDIAN MODEL AVAILABLE HOTEL & HOME ★ SAFE AND SECURE HIGH CLASS SERVICE AFFORDABLE RATE ★ 100% SATISFACTION,UNLIMITED ENJOYMENT. ★ All Meetings are confidential and no information is provided to any one at any cost.
★ EXCLUSIVE Profiles Are Safe and Consensual with Most Limits Respected
★ Service Available In: - HOME & 24x7 :: 3 * 5 *7 *Star Hotel Service .In Call & Out call
Services :
★ A-Level (5 star escort)
★ Strip-tease
★ BBBJ (Bareback Blowjob)Receive advanced sexual techniques in different mode make their life more pleasurable.
★ Spending time in hotel rooms
★ BJ (Blowjob Without a Condom)
★ Completion (Oral to completion)
★ Covered (Covered blowjob Without a Condom)Chandigarh Call Girls❤️Chandigarh Escort Service☎️9815457724☎️ Call Girl service in Chandigarh☎️ C...

❤️Chandigarh Escort Service☎️9815457724☎️ Call Girl service in Chandigarh☎️ C...Rashmi Entertainment
Dernier (20)
❤️Chandigarh Escorts Service☎️9815457724☎️ Call Girl service in Chandigarh☎️ ...

❤️Chandigarh Escorts Service☎️9815457724☎️ Call Girl service in Chandigarh☎️ ...
💸Cash Payment No Advance Call Girls Nagpur 🧿 9332606886 🧿 High Class Call Gir...

💸Cash Payment No Advance Call Girls Nagpur 🧿 9332606886 🧿 High Class Call Gir...
💸Cash Payment No Advance Call Girls Surat 🧿 9332606886 🧿 High Class Call Girl...

💸Cash Payment No Advance Call Girls Surat 🧿 9332606886 🧿 High Class Call Girl...
science quiz bee questions.doc FOR ELEMENTARY SCIENCE

science quiz bee questions.doc FOR ELEMENTARY SCIENCE
Call Now ☎ 8868886958 || Call Girls in Chandigarh Escort Service Chandigarh

Call Now ☎ 8868886958 || Call Girls in Chandigarh Escort Service Chandigarh
💞 Safe And Secure Call Girls Mysore 🧿 9332606886 🧿 High Class Call Girl Servi...

💞 Safe And Secure Call Girls Mysore 🧿 9332606886 🧿 High Class Call Girl Servi...
❤️ Zirakpur Call Girl Service ☎️9878799926☎️ Call Girl service in Zirakpur ☎...

❤️ Zirakpur Call Girl Service ☎️9878799926☎️ Call Girl service in Zirakpur ☎...
Gorgeous Call Girls In Pune {9xx000xx09} ❤️VVIP ANKITA Call Girl in Pune Maha...

Gorgeous Call Girls In Pune {9xx000xx09} ❤️VVIP ANKITA Call Girl in Pune Maha...
Ulhasnagar Call girl escort *88638//40496* Call me monika call girls 24*

Ulhasnagar Call girl escort *88638//40496* Call me monika call girls 24*
Indore Call Girl Service 📞9235973566📞Just Call Inaaya📲 Call Girls In Indore N...

Indore Call Girl Service 📞9235973566📞Just Call Inaaya📲 Call Girls In Indore N...
💸Cash Payment No Advance Call Girls Bhopal 🧿 9332606886 🧿 High Class Call Gir...

💸Cash Payment No Advance Call Girls Bhopal 🧿 9332606886 🧿 High Class Call Gir...
❤️Chandigarh Escort Service☎️9815457724☎️ Call Girl service in Chandigarh☎️ C...

❤️Chandigarh Escort Service☎️9815457724☎️ Call Girl service in Chandigarh☎️ C...
💸Cash Payment No Advance Call Girls Kolkata 🧿 9332606886 🧿 High Class Call Gi...

💸Cash Payment No Advance Call Girls Kolkata 🧿 9332606886 🧿 High Class Call Gi...
Low Rate Call Girls Jaipur {9521753030} ❤️VVIP NISHA CCall Girls in Jaipur Es...

Low Rate Call Girls Jaipur {9521753030} ❤️VVIP NISHA CCall Girls in Jaipur Es...
Low Rate Call Girls Nagpur {9xx000xx09} ❤️VVIP NISHA Call Girls in Nagpur Mah...

Low Rate Call Girls Nagpur {9xx000xx09} ❤️VVIP NISHA Call Girls in Nagpur Mah...
❤️Chandigarh Escort Service☎️9814379184☎️ Call Girl service in Chandigarh☎️ C...

❤️Chandigarh Escort Service☎️9814379184☎️ Call Girl service in Chandigarh☎️ C...
Premium Call Girls Bangalore {9179660964} ❤️VVIP POOJA Call Girls in Bangalor...

Premium Call Girls Bangalore {9179660964} ❤️VVIP POOJA Call Girls in Bangalor...
💸Cash Payment No Advance Call Girls Hyderabad 🧿 9332606886 🧿 High Class Call ...

💸Cash Payment No Advance Call Girls Hyderabad 🧿 9332606886 🧿 High Class Call ...
❤️Zirakpur Escorts☎️7837612180☎️ Call Girl service in Zirakpur☎️ Zirakpur Cal...

❤️Zirakpur Escorts☎️7837612180☎️ Call Girl service in Zirakpur☎️ Zirakpur Cal...
Top 20 Famous Indian Female Pornstars Name List 2024

Top 20 Famous Indian Female Pornstars Name List 2024
Japan medical device approval chart - Emergo
- 1. The regulatory process for medical devices You may now begin marketing your device in Japan. Approvals do not expire.** After submission, certification or approval, MAH/D-MAH must submit Import Notification to Regional Bureau of Health and Welfare. In the meantime, Reimbursement application should be filed with Economic Affairs Division of MHLW if applicable. No certificate issued by PMDA. Pre-Market Certificate issued by RCB. Pre-Market Approval certificate issued by MHLW. QMS Conformance certificate issued by PMDA or RCB. Some Class I devices require QMS conformity assessment by PMDA. QMS audit by Registered Certification Body (RCB). QMS audit by PMDA. On-site audits are typically required for “New” devices with no existing JMDN code, Class IV devices, and those requiring clinical investigations. Submit Pre-Market Submission to Pharmaceutical and Medical Devices Agency (PMDA).* All documents must be in Japanese. Submit Pre-Market Certification application to a Registered Certified Body (RCB) authorized to issue certifications. All documents must be in Japanese. Prepare Pre-Market Approval application as well as registration dossier in Summary Technical Document (STED) format. Submit documents to PMDA. All documents must be in Japanese. 5000-0115 * MHLW Ordinance #169 documentation is not required in most PMS applications. ** Device registrations do not expire. However, to maintain QMS certification periodic audits will be conducted. The schedule will be determined by the RCB or PMDA. This is a simplified overview of the process. The PMDA may choose to audit your submission and request more documents, which will add time to your approval. © 2014 Emergo – You are welcome to publish this chart on your website, or copy it for use in presentations or other materials if it is not cropped in any way. Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 01/2015. EmergoGroup.com/japan JPJapan Determine classification of your device according to the Pharmaceutical and Medical Device Law (PMDL) and Japanese Medical Device Nomenclature (JMDN) codes. General Class I Medical Devices Specified Controlled Class II Medical Devices Controlled Class II Medical Devices Highly Controlled Class III Medical Devices Highly Controlled Class IV Medical Devices Appoint Marketing Authorization Holder (MAH or D-MAH) to manage your device registration in Japan. Your MAH or D-MAH will control your device registration. Manufacturers must register their facilities with the PMDA. Implement Quality Management System (QMS) which complies with the PMDL and Ministry of Health, Labour and Welfare (MHLW) Ordinance #169. Ordinance #169 is based on ISO 13485.
- 2. The regulatory process for medical devices Device classification in Japan How long you should expect to wait after submission until approval is granted. (See note 1) Validity period for device registrations. (See note 2) Registration renewal should be started this far in advance. (See note 2) Complexity of the registration process for this classification. (See note 3) Overall cost of gaining regulatory approval. (See note 4) CLASS I General Medical Devices 1 month Does not expire 1 month Simple Complex Low High CLASS II Specified Controlled Medical Devices 3-5 months Does not expire 1 month Simple Complex Low High CLASS II Controlled Medical Devices 7-9 months Does not expire 1 month Simple Complex Low High CLASS III Highly Controlled Medical Devices 9-11 months Does not expire 1 month Simple Complex Low High CLASS IV Highly Controlled Medical Devices 13-16 months Does not expire 1 month Simple Complex Low High JPJapan EmergoGroup.com/japan NOTE 1: The time frames shown above are typical for the majority of medical device submissions but assume that your device does not contain animal tissue, medicinal substances or employ entirely novel technology. Your length of approval will depend on the quality and completeness of your technical documentation and how much time you take to address additional information requests from authorities after submission. YOUR SUBMISSION(S) MAY TAKE MORE TIME THAN WHAT IS SHOWN ABOVE. NOTE 2: Device registration does not expire, however you must pay Foreign Manufacturer Registration (FMR) renewal fees. Failure to renew FMR will result in your permission to market your device being revoked. NOTE 3: Our rating of the complexity of the registration process is based on our experience and the opinion of nearly 1,000 QA/RA professionals worldwide who were asked to rate the difficulty of registering a device in each country in January 2014. The European CE Marking process is considered the mid-point to which all other markets are compared. NOTE 4: Low = Less than US$5000; Midpoint = US$15000-$30000: High = More than US$50000. Overall cost includes registration application fees, product testing, in- country representation, submission preparation consulting and translation of registration documents but not IFU. Does not include cost of implementing or auditing a quality management system compliant with ISO 13485 or US FDA 21 CFR Part 820, if applicable. © 2014 Emergo Group – You are welcome to publish this chart on your website, or copy it for use in presentations or other materials if it is not cropped in any way. Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 01/2015.
