Atomic Strucutre
•Télécharger en tant que PPTX, PDF•
0 j'aime•293 vues
Protons have a positive charge and determine atomic number, neutrons have no charge and equal mass to protons, electrons have a negative charge and equal number to protons in neutral atoms. Atomic symbols represent the atomic number Z of protons and mass number A of protons plus neutrons. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons, giving them different mass numbers.
Signaler
Partager
Signaler
Partager
Recommandé
Recommandé
Contenu connexe
Tendances (9)
Elements, compounds, molecules and subatomic particles 

Elements, compounds, molecules and subatomic particles
En vedette
En vedette (13)
Lesson 4 Ionizing Radiation | The Harnessed Atom (2016)

Lesson 4 Ionizing Radiation | The Harnessed Atom (2016)
13 - Nuclear Magnetic Resonance Spectroscopy - Wade 7th

13 - Nuclear Magnetic Resonance Spectroscopy - Wade 7th
IB Chemistry on Nuclear Magnetic Resonance, Chemical Shift and Splitting Pattern

IB Chemistry on Nuclear Magnetic Resonance, Chemical Shift and Splitting Pattern
Power point presentation of animal cell and plant cell

Power point presentation of animal cell and plant cell
Atomic Spectroscopy: Basic Principles and Instruments

Atomic Spectroscopy: Basic Principles and Instruments
Plus de gbsliebs2002
Plus de gbsliebs2002 (20)
Atomic Strucutre
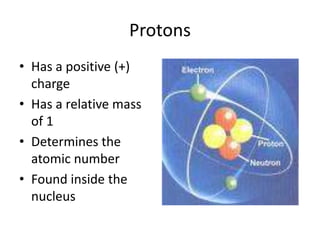
- 1. Protons Has a positive (+) charge Has a relative mass of 1 Determines the atomic number Found inside the nucleus
- 2. Neutrons Has a relative mass of 1 Found inside the nucleus Charge is = to zero Equal to the # of protons in a neutral atom
- 4. Has a relative mass of 0 (zero)
- 5. Found outside the nucleus
- 7. Isotopes Isotopes are two atoms of the same element Same atomic number Different mass numbers Number of neutrons is A-Z Number of neutrons differs between isotopes