Stoichiometric Calculations
•Télécharger en tant que PPTX, PDF•
3 j'aime•5,447 vues
Signaler
Partager
Signaler
Partager
Recommandé
Contenu connexe
Tendances
Tendances (20)
En vedette
En vedette (20)
Similaire à Stoichiometric Calculations
Similaire à Stoichiometric Calculations (20)
526128650-Limiting-Reactants-and-the-Product-Formed.pdf

526128650-Limiting-Reactants-and-the-Product-Formed.pdf
526128650-Limiting-Reactants-and-the-Product-Formed.pdf

526128650-Limiting-Reactants-and-the-Product-Formed.pdf
Plus de gbsliebs2002
Plus de gbsliebs2002 (20)
Dernier
Dernier (20)
Repurposing LNG terminals for Hydrogen Ammonia: Feasibility and Cost Saving

Repurposing LNG terminals for Hydrogen Ammonia: Feasibility and Cost Saving
Elevate Developer Efficiency & build GenAI Application with Amazon Q

Elevate Developer Efficiency & build GenAI Application with Amazon Q
TrustArc Webinar - Unlock the Power of AI-Driven Data Discovery

TrustArc Webinar - Unlock the Power of AI-Driven Data Discovery
Apidays New York 2024 - Accelerating FinTech Innovation by Vasa Krishnan, Fin...

Apidays New York 2024 - Accelerating FinTech Innovation by Vasa Krishnan, Fin...
Introduction to Multilingual Retrieval Augmented Generation (RAG)

Introduction to Multilingual Retrieval Augmented Generation (RAG)
Cloud Frontiers: A Deep Dive into Serverless Spatial Data and FME

Cloud Frontiers: A Deep Dive into Serverless Spatial Data and FME
Apidays New York 2024 - APIs in 2030: The Risk of Technological Sleepwalk by ...

Apidays New York 2024 - APIs in 2030: The Risk of Technological Sleepwalk by ...
Apidays New York 2024 - The value of a flexible API Management solution for O...

Apidays New York 2024 - The value of a flexible API Management solution for O...
Mcleodganj Call Girls 🥰 8617370543 Service Offer VIP Hot Model

Mcleodganj Call Girls 🥰 8617370543 Service Offer VIP Hot Model
How to Troubleshoot Apps for the Modern Connected Worker

How to Troubleshoot Apps for the Modern Connected Worker
Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood

Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood
Strategize a Smooth Tenant-to-tenant Migration and Copilot Takeoff

Strategize a Smooth Tenant-to-tenant Migration and Copilot Takeoff
Cloud Frontiers: A Deep Dive into Serverless Spatial Data and FME

Cloud Frontiers: A Deep Dive into Serverless Spatial Data and FME
Finding Java's Hidden Performance Traps @ DevoxxUK 2024

Finding Java's Hidden Performance Traps @ DevoxxUK 2024
EMPOWERMENT TECHNOLOGY GRADE 11 QUARTER 2 REVIEWER

EMPOWERMENT TECHNOLOGY GRADE 11 QUARTER 2 REVIEWER
Stoichiometric Calculations
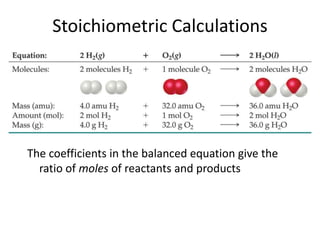
- 1. Stoichiometric Calculations The coefficients in the balanced equation give the ratio of moles of reactants and products
- 2. Stoichiometric Calculations From the mass of Substance A you can use the ratio of the coefficients of A and B to calculate the mass of Substance B formed (if it’s a product) or used (if it’s a reactant)
- 3. Stoichiometric Calculations C6H12O6 + 6 O2 6 CO2 + 6 H2O Starting with 1.00 g of C6H12O6… we calculate the moles of C6H12O6… use the coefficients to find the moles of H2O… and then turn the moles of water to grams
- 4. Sample #1 CH4 +2O22H2O + CO2 How many moles of CH4 are needed to make 13 moles of water?
- 5. Sample #2 CH4 +2O22H2O + CO2 How many moles of water are made from 10g of Oxygen?
- 6. Sample #3 CH4 +2O22H2O + CO2 How many grams of water are produced from 100 moles of CH4?
- 7. Sample #4 CH4 +2O22H2O + CO2 How many grams of Oxygen are needed in order to produce 200 g of carbon dioxide?
- 8. Limiting Reactants The limiting reactant is the reactant present in the smallest stoichiometric amount
- 9. Limiting Reactants The limiting reactant is the reactant present in the smallest stoichiometric amount In other words, it’s the reactant you’ll run out of first (in this case, the H2)
- 10. Limiting Reactants In the example below, the O2 would be the excess reagent
- 11. Theoretical Yield The theoretical yield is the amount of product that can be made In other words it’s the amount of product possible as calculated through the stoichiometry problem This is different from the actual yield, the amount one actually produces and measures
- 12. Percent Yield Actual Yield Theoretical Yield Percent Yield = x 100 A comparison of the amount actually obtained to the amount it was possible to make