UBD Learning Plan
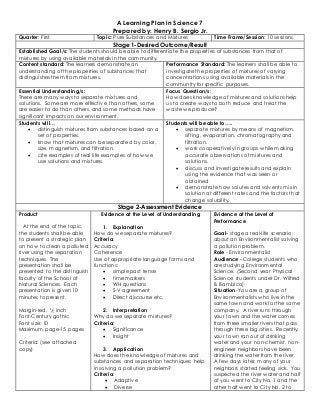
- 1. A Learning Plan in Science 7 Prepared by: Henry B. Sergio Jr. Quarter: First Topic: Pure Substances and Mixtures Time Frame/Session: 10 sessions Stage 1-Desired Outcome/Result Established Goal/s: The students should be able to differentiate the properties of substances from that of mixtures by using available materials in the community. Content standard: The learners demonstrate an understanding of the properties of substances that distinguishes them from mixtures. Performance Standard: The learners shall be able to investigate the properties of mixtures of varying concentrations using available materials in the community for specific purposes. Essential Understanding/s: There are many ways to separate mixtures and solutions. Some are more effective than others, some are easier to do than others, and some methods have significant impacts on our environment. Focus Question/s: How does knowledge of mixtures and solutions help us to create ways to both reduce and treat the waste we produce? Students will… distinguish mixtures from substances based on a set of properties. know that mixtures can be separated by color, size, magnetism, and filtration. cite examples of real life examples of how we use solutions and mixtures. Students will be able to….. separate mixtures by means of magnetism, sifting, evaporation, chromatography and filtration. work cooperatively in groups while making accurate observations of mixtures and solutions. discuss and investigate results and explain using the evidence that was seen or obtained demonstrate how solutes and solvents mix in solution at different rates and the factors that change solubility. Stage 2-Assessment Evidence Product At the end of the topic, the students shall be able to present a strategic plan on how to clean a polluted river using the separation techniques. The presentation shall be presented to the distinguish faculty of the School of Natural Sciences. Each presentation is given 10 minutes to present. Margin-red, ½ inch Font-Century gothic Font size: 10 Maximum page-15 pages Criteria: (see attached copy) Evidence at the Level of Understanding 1. Explanation How do we separate mixtures? Criteria: Accuracy Coherence Use of appropriate language forms and functions: simple past tense time markers WH questions S-V agreement Direct discourse etc. 2. Interpretation Why do we separate mixtures? Criteria: Significance Insight 3. Application How does the knowledge of mixtures and substances and separation techniques help in solving a pollution problem? Criteria: Adaptive Diverse Evidence at the Level of Performance Goal- stage a real-life scenario about an Environmentalist solving a pollution problem. Role - Environmentalist Audience –College students who are studying Environmental Science. (Second year Physical Science students under Dr. Wilfred B. Bambico) Situation- You are a group of Environmentalists who live in the same town and work for the same company. A river runs through your town and the water comes from three smaller rivers that pass through three big cities. Recently, your town ran out of drinking water and your non-chemist, non-engineer neighbors have been drinking the water from the river. A few days later, many of your neighbors started feeling sick. You suspected the river water and half of you went to City No. 1 and the other half went to City No. 2 to
- 2. Effective 4. Perspective Do all the separation techniques separate a mixture? Criteria: Critical in analytical sense Insightful Revealing 5. Empathy What are some cases at home that we need to separate a certain mixture? Criteria: Perceptive Open-mindedness Sensitive 6. Self-knowledge How can I use my knowledge about mixtures and substances in solving pollution? Criteria: Reflective Responsive Efficient Effective see what is in their river. In City No. 1, you saw that a factory is right next to the river and they have been putting chemicals in the river. In City No. 2, the other half of you saw that the water appears to be clean but have a lot of sand in it. You are now asked to come up with a way to separate the chemicals from the water in City No. 1 and the sand from the water in City No. 2. A separate group of Environmentalists went to City No. 3 and found that ethanol and salt are in their river water. That means a mixture of water, ethanol (isopropanol), acetone, salt, and sand is in the river that runs through your town. Remember, your town is out of drinking water, so you must clean the river water and separate all the chemicals, salt, and sand from the river water to have drinking water! Performance- Present a role play on how you will solve the situation integrating the different separation techniques. Standard- (See attached copy) Stage 3-Learning Plan Teaching/Learning Sequence: A. Explore 1. Define Substances and Mixtures and give 2 examples each. A substance is composed of only a pure element. Example is Hydrogen and Oxygen. A mixture is composed of two or more substances or mixtures or combination of both. Example is juice and the air. 2. Sing a line from the song “We are the World.” “We are the world, we are the children. We are the ones who make a brighter day so let’s start giving. There’s a choice we’re making, we’re saving our own lives. It’s true we’ll make a better day just you and me.” In what way should we act as humans? What does it mean by being one? We should act according to the norms of our society and not merely on our own will. Being one means there’s no one on top but it is a congregation of all from different place, race, gender, religion, social status and skin color. 3. Process the answer of the students.
- 3. Being one teaches us unity and unity teaches us that we are composed of not just one but variety of members. This leads us to our lesson which is Mixtures and Substances. 4. Through a vertical bullet list, explain to the class the difference of a mixture and a solution with examples. Pure substances •contains only one type of particle. •Substances don't usually occur in their pure form in nature, so in order to obtain pure substances, people must refine raw materials. •Examples: Gold, water, lead etc.. Mixtures •Mixtures are substances that consist of combinations of two or more pure substances, or different particles. •Mixtures can be in the form of solids, liquids, and/or gases, in any combination. •Examples: Air,concrete, juice etc. Examples of Substances: Examples of Mixtures: Lead Gold Water Air Juice Concrete 5. Discuss on the Particle Theory of Matter. The Particle Theory of Matter states that: Matter is made up of tiny particles (Atoms & Molecules) 6. Post on the board a picture of the formation of the substances and mixtures. What can you say about the pictures?
- 4. 7. Group activity 2. Each group must come up with a presentation about how mixtures and substances are formed using only the members of the group 8. Ask the focus question. Substances are formed from only 1 particle whereas mixtures are formed from 2 or more substances. 1. Group the class into 5 groups with 10 members each. 3. They are given 3 minutes each only to present. How does knowledge of mixtures and solutions help us to create ways to both reduce and treat the waste we produce? laboratory waste that may present chemical hazards, as well as those multihazardous wastes that contain some combination of chemical, radioactive, and biological hazards. The best strategy for managing laboratory waste aims to maximize safety and minimize environmental impact, and considers these objectives from the 9. Clarify misconceptions of the students. Knowledge of mixtures and solutions is for the management and ultimate disposal of 10. From the given examples of known things at home, a student picks one and share to the class if it’s a substance or a mixture and what composes it. time of purchase. -The solvent in a solution disappears when mixed with the solute. -Solutes absorb the solvents in solution. When mixing solutions a chemical change will always occur to change solution to another liquid
- 5. B. Firm-up 1. From the pictures posted on the board, the students should determine what kind of separation technique was used. Evaporation Magnetism 2. From the posted pictures of the different separation techniques, define each using the vertical chevron list. I choose water. It is a substance. It is made up of the particles of water which is hydrogen and oxygen. Sifting Filtration Chromatography
- 6. 3. Post on the board a problem. 4. Administer a class activity: Divide the class into 5 groups with 8 members each. Each group will be assigned on a separation technique and perform the principle in each separation technique to separate the gravel and the sand. Distribute the materials to each group. Common material for each group is the mixture of sand and gravel. Group A-sand and a gravel, Group C-seawater, Group D-dye, Group E-water and Group B-and sulfur in iron filings. 5. Give to each group the materials to be used. Group A: Sifting sifter magne-tism •use of magnet to separate metals from non-metals. Sifting •a method in which you use the property of size to separate mixtures Evapora-tion •liquid portion of the solution is allowed to evaporate, leaving the solute behind Filtration •is a method that uses the property of the boiling point to separate two components of a solution Chroma-tography •is a method that uses the property of thea bsorption rate to separate different -coloured substances from a solution. Henry has a lot of mixtures in his house. He wants to separate those to easily dispose them and gain profit from it. How can he separate the following mixtures: Sand and a gravel, seawater, dye, water and sand, and sulfur in iron filings?
- 7. Group B: Magnetism horseshoe magnet Group C. Evaporation evaporating dish alcohol lamp Group D: Chromatography beaker with water paper stick Group E: Filtration flask with water funnel filter paper Each group will try to separate the sand and gravel using the method assigned to them. The group also demonstrates the method assigned to them. 6. Outcome of the Activity
- 8. Group A-the sand and gravel separated. Group B- the iron filings separated from the sulfur Group C- the water evaporated leaving behind salt. Group D- the dye separated into different colors like green, blue, yellow and red. Group E- the sand was left behind the filter paper. 7. How does the knowledge of mixtures and substances and separation techniques help in solving a pollution problem? Chemicals we use to wash our homes, cars and even our bodies get washed down the drain and into the sewer system, but they often end up in the water supply. These chemicals aren't good for the plants and animals that make up our ecosystems, and they aren't healthy for human consumption, either. Whenever possible, use natural, healthy alternatives to chemicals. For example, instead of using a heavy-duty cleaner to scrub your bathroom or kitchen, use a mixture of vinegar and water or a baking soda and salt paste. These natural household supplies get the job done just as well, and they won't pollute the water when you wash them down the drain. Try making your own laundry detergent and dish soap. If you don't have the time, buy detergent made with all-natural ingredients. When you can't find a good alternative to a toxic item, use the least amount you can get away with and still get the job done.
- 9. 8. Why do we want to separate mixtures? All the way back to Ancient History, industrious humans have separated mixtures in order to obtain the specific substances that they need. One example of this is extracting metal from ore in order to make 9. Name the techniques which are suitable for separating the following mixture. 10 points a. To obtain drinking water from muddy water- Evaporation/Filtration/Distillation b. To separate petrol from crude oil- Distillation c. To remove leaves from a swimming pool.- Sifting d. To obtain pure sugar from a solution.- Evaporation e. To determine whether the coloring in a fruit juice is a single substance or a mixture of colored substance- Chromatography 10. Tell the class that they will have a role play about solving pollution and a strategic plan on water pollution. C. Reflect and Understand 1. tools and weapons. How does knowledge of mixtures and solutions help us to create ways to both reduce and treat the waste we produce?
- 10. Knowledge of mixtures and solutions is for the management and ultimate disposal of laboratory waste 2. What are the different methods of separation techniques? The methods of separation techniques are the following: magnetism, evaporation, distillation, chromatography, and sifting. The use of each depends on the kind of mixture. 3. Group the class into 5 groups with 10 members. The students choose chromatography. Instead of color, they will represent it as the human race. And after series of investigations, the group separates the girls in the group from the boys. Criteria: Group discipline-10 points Accuracy of the presentation-15 points Total: 25 points 4. Differentiate mixtures from substances. 5. that may present chemical hazards, as well as those multihazardous wastes that contain some combination of chemical, radioactive, and biological hazards. The best strategy for managing laboratory waste aims to maximize safety and minimize environmental impact, and considers these objectives from the time of purchase. Substance is an element or a compound (two or more elements that have reacted chemically in a fixed proportion by mass).We can separate the various constituents of a compound substance by a chemical process (reaction). Mixture is formed when two or more substances are mixed in any proportion. The constituents of a mixture can be separated by a physical process. Mixtures Substances Which separation method — magnetism, filtration or sifting, evaporation, distillation, or chromatography — would you recommend to divide the components of mixtures in the following examples? Defend your answer. 6 points 1. A chef is preparing stew and finds it is too watery. Without adding anything, how can the chef separate some of the water from the mixture? 2. A person is allergic to the yellow dying agent used in manufacturing certain candy coatings. Although the coatings appear as one color, the colors are often made from a combination of dyes. What separation method can be used to determine if a yellow dying agent was used?
- 11. 1. 2. Chromatography 6. Identify the following statements to be TRUE or FALSE. Evaporation a. In filtration, the filtrate is always a pure liquid. True/False b. Drinking water can only be obtained from seawater by distillation. True/False c. The fractional distillation of miscible liquids is only possible if the liquids have different boiling points. True/False d. Paper chromatography is a physical method for separating mixtures. True/False e. Mixtures have fixed melting and boiling points. True/False 7. Experiment on Paper chromatography a. Tell the class to be on their respective group and areas. b. Distribute to them their work sheets and materials to be used. Smarties paint brush beaker chromatography paper ink stick c. Work period Bend a paper clip so that it is straight with a hook at one end. Push the straight end of the paper clip into the bottom of the rubber stopper. Next, you hang a thin strip of filter paper on the hooked end of the paper clip. Insert the paper strip into the test tube. The paper should not touch the sides of the test tube and should almost touch the bottom of the test tube. Now you will remove the paper strip from the test tube. Draw a solid 5-mm-wide band about 25 mm from the bottom of the paper, using the black felt-tip pen. Use a pencil to draw a line across the paper strip 10 cm above the black band.
- 12. Pour about 2 mL of water into the test tube. The water will act as a solvent. Put the filter paper back into the test tube with the bottom of the paper in the water and the black band above the water. Observe what happens as the liquid travels up the paper. Record the changes you see. When the solvent has reached the pencil line, remove the paper from the test tube. Measure how far the solvent traveled before the strip dries. Finally, let the strip dry on the desk. With the metric ruler, measure the distance from the starting point to the top edge of each color. Record this data in a data table. Calculate a ratio for each color by dividing the distance the color traveled by the distance the solvent traveled. 8. Present the expected outcome. Before chromatography: After chromatography: 9. Why did the inks separate? The inks separated because the black ink was a mixture of different pigments with different molecular characteristics. These differences allow for different rates of absorption by the filter paper. The inks separated because the black ink was a mixture of different pigments with different molecular characteristics. These differences allow for different rates of absorption by the filter paper. 10. Select the best answer. Letters only. 1. Which is the best way to get salt from salty water? a. evaporation b. filtration c. distillation 2. Pure water can be separated from inky water by simple distillation. This is because: a. water and ink have different boiling points. b. water evaporates leaving the ink particles behind. c. ink evaporates leaving the water behind. 3. What is the correct order for obtaining salt from a mixture of sand and salt? a. dissolving in water - filtration - evaporation b. evaporation - filtration - dissolving in water c.filtration - dissolving in water - evaporation D. Transfer 1. Product At the end of the topic, the students shall be able to present a strategic plan on how to clean a polluted river using the separation techniques. The presentation shall be presented to the distinguish faculty of the School of Natural Sciences. Each presentation is given 10 minutes to present. Margin-red, ½ inch Font-Century gothic Font size: 10 Maximum page-15 pages
- 13. 2. The compilation should contain a plan on how to use separation techniques in cleaning polluted water. 3. It must follow the cycle of strategic plan. *See attached copy of criteria 4. Performance Goal- stage a real-life scenario about an Environmentalist solving a pollution problem. Role - Environmentalist Audience –College students who are studying Environmental Science. (Second year Physical Science students under Dr. Wilfred B. Bambico) Situation- You are a group of Environmentalists who live in the same town and work for the same company. A river runs through your town and the water comes from three smaller rivers that pass through three big cities. Recently, your town ran out of drinking water and your non-chemist, non-engineer neighbors have been drinking the water from the river. A few days later, many of your neighbors started feeling sick. You suspected the river water and half of you went to City No. 1 and the other half went to City No. 2 to see what is in their river. In City No. 1, you saw that a factory is right next to the river and they have been putting chemicals in the river. In City No. 2, the other half of you saw that the water appears to be clean but have a lot of sand in it. You are now asked to come up with a way to separate the chemicals from the water in City No. 1 and the sand from the water in City No. 2. A separate group of Environmentalists went to City No. 3 and found that ethanol and salt are in their river water. That means a mixture of water, ethanol (isopropanol), acetone, salt, and sand is in the river that runs through your town. Remember, your town is out of drinking water, so you must clean the river water and separate all the chemicals, salt, and sand from the river water to have drinking water! Performance- Present a role play on how you will solve the situation integrating the different separation techniques. Standard: (See attached copy) Resources: Hadsal, A.S.(2008).Exploring science and technology II. Diwa learning system, Inc. Mendoza, E.A. et al (1997).Science and technology: Chemistry. Phoenix Publishing House Inc. Boundless. “Substances and Mixtures.” Boundless Chemistry. Boundless, 29 Oct. 2014. Retrieved 14 Nov. 2014 from https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/introduction-to- chemistry-1/classification-of-matter-27/substances-and-mixtures-179-3707/ Materials Needed: • Matter chart diagram
- 14. • Pictures showing a mixture and a substance • Water in a glass • ½ teaspoon of a mango powder juice. • Common things seen at home (Oil, water, butter, rock etc..) • Bucket • Sand • Gravel stones • Sifter • Beakers • Paper • Water • Flask • Alcohol lamp • Evaporating dish • Magnet • Sifter • Water • Filter paper Rubric for Presenting a Role Play Assessment Criteria Indicators for the Levels of Performance Score Appropriateness 1 point 3 points 5 points The presented solutions to the problem are not appropriate to the scenario. The presented solutions to the problem are fairly appropriate to the scenario The presented solutions to the problem are very appropriate to the scenario. Delivery The dialogues are poorly delivered The dialogues are properly delivered. All of the dialogues are well delivered. Consistency and organization The flow of events and ideas is inconsistent and disorganized. The flow of events and ideas is consistent but it needs to be more organized. The flow of events and ideas is consistent and well organized. Total: Criteria for Evaluating the Strategic Plan Presentation Criteria Points Score Content: Development of Context, Depth of Analysis, Accuracy, Depth of Insights 30 Quality of Graphics 5 Correct Format for Tables and Figures 10 Effective Use of Materials 10 Delivery of Presentation (speed, clarity of articulation, eye contact) 5 Strength of Transitions Between Speakers 5 Coordination with Other Presentations by Group Members 5 PowerPoint Effectiveness 5 Audience Engagement 5 Ability to Answer Questions 20 Total: 100
