Somatosensation: Revision Notes
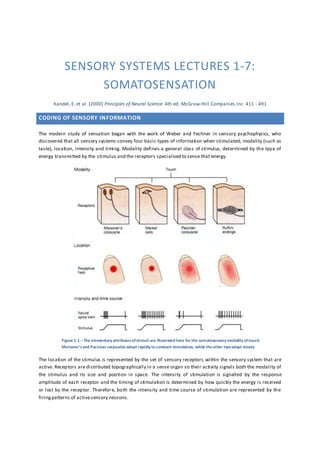
- 1. SENSORY SYSTEMS LECTURES 1-7: SOMATOSENSATION Kandel, E. et al. (2000) Principles of Neural Science 4th ed. McGraw-Hill Companies Inc. 411 - 491 CODING OF SENSORY INFORMATION The modern study of sensation began with the work of Weber and Fechner in sensory psychophysics, who discovered that all sensory systems convey four basic types of information when stimulated; modality (such as taste), location, intensity and timing. Modality defines a general class of stimulus, determined by the type of energy transmitted by the stimulus and the receptors specialised to sense that energy. Figure 1.1 –The elementary attributesofstimuli are illustrated here for the somatosensory modality oftouch. Meissner’s and Pacinian corpuscles adopt rapidly to constant stimulation, while theother twoadapt slowly The location of the stimulus is represented by the set of sensory receptors within the sensory system that are active. Receptors are distributed topographically in a sense organ so their activity signals both the modality of the stimulus and its size and position in space. The intensity of stimulation is signalled by the response amplitude of each receptor and the timing of stimulation is determined by how quickly the energy is received or lost by the receptor. Therefore, both the intensity and time course of stimulation are represented by the firingpatterns of activesensory neurons.
- 2. 2 Coding of Sensory Information As well as the traditional five senses (vision, hearing, touch, taste and smell), we also consider the somatic senses of pain, temperature, itch and proprioception (posture and movement of body parts) as well as the vestibular senseof balance(the position of the body in the gravitational field). In 1826, Johannes Müller provided an early insight into the neuronal basis of sensation with his “laws of specific sense energies”. He proposed that modality is the property of the sensory nerve fibre and each fibre is activated primarily by a certain type of stimulus which makes specific connections to structures in the CNS whose activity gives rise to specific sensations. Thus Müller’s laws of specific sense energies identified the most important mechanismfor neural codingof stimulus modality. MODALITY IS ENCODED BY A LABELLED LINE CODE The electrical signal produced at sensory receptors is termed the receptor potential. The amplitude and duration of this potential are directly related to the intensity and time course of stimulation of the particular receptor. The process by which specific stimulus energy is converted into an electrical signal is termed stimulus transduction. Receptors are morphologically specialised to transducer specific forms of energy. Each receptor has a specialised anatomical region where stimulus transduction occurs, and most sensory receptors are optimally selectivefor a singlestimulus energy, called receptor specifity. The specifity of a response in receptors underlies the labelled line code, the most important mechanism for stimulus modality. Receptors are selective for a particular type of stimulus energy, so the axons act as a modality specific line of communication. Excitation of a particular sensory neuron, whether naturally or artificially, elicits the same sensation. Each class of sensory systems makes contact with distinctive structure sin the CNS, at least in the early stages of information processing, thus a sense is detected due to activation of a particular CNS structure. Modality is therefore represented by the ensemble of neurons connected to a specific class of receptors, called sensory systems, which comprise the somatosensory system, visual system, auditory system, vestibular system,olfactory system and gustatory system. Humans have four classes of receptors; mechanical, chemical, thermal and electromagnetic. The mechanoreceptors of the somatosensory system mediate touch, proprioceptive sensations (like muscle stretch or contraction), and the sense of joint position, whereas mechanoreceptors in the inner ear mediate hearing and the sense of balance. Chemoreceptors are involved in the senses of pain, itch, taste and smell. Thermoreceptors in the skin sense temperatures within and outside the body as we come into contact with them and the only electromagnetic receptors humans possess arethe photoreceptors in the retina. RECEPTORS TRANSDUCE SPECIFIC TYPES OF ENERGY INTO AN ELECTRICAL SIGNAL Mechanoreceptors sense physical deformation in the tissue they reside, and pressure is transduced into electrical energy by the physical impact on cation channels in the membrane that are linked to the cytoskeleton. Mechanical stimulation deforms the receptor membrane, opening stretch-sensitive ion channels and increasing ion conductances that depolarise the receptor. (The depolarising receptor potential is therefore similar in mechanism to the excitatory postsynaptic potential.) The amplitude of the receptor potential is proportional to the stimulus intensity; by opening more ion channels for a longer period of time, strong pressure produces greater depolarisation, while removal of the stimulus relieves the chemical stress on the receptor membrane and causes stretch-sensitive channels to close. Mechanoreceptors in the inner ear respond to the bending of sensory cilia on the apical membrane, when the sensory hairs are deflected in one direction by sounds of the appropriate frequency, the receptor cell depolarises, whereas deflection in the oppositedirection hyperpolarises thereceptor call.
- 3. Coding of Sensory Information 3 Receptor potentials in chemoreceptors and photoreceptors are generated by intracellular second messengers, which are activated through GPCRs, and may produce conductance changes either locally or at remote sites. Chemoreceptors usually respond to the appropriate ligand with a depolarising potential. Photoreceptors, by contrast, respond to light through hyperpolarisation. The great advantage or secondary-messenger mediated mechanisms is thatthe sensory signal becomes amplified. EACH RECEPTOR RESPONDS TO A NARROW RANGE OF STIMULUS ENERGY Each of the major modalities has several submodalities; for example, taste can be sweet, sour, salty or bitter, objects that we see differ in colour, shape and movement, and touch has qualities of temperature, texture and rigidity. These submodalities exist because each class of receptors contains a variety of specialised receptors that respond to a limited range of stimulus energies. Receptors behave as a filter for a specific bandwidth; individual photoreceptors are only sensitive to a small part of the light spectrum. Since receptors are tuned to an adequate stimulus, we can plot a tuning curve for each receptor based on physiological experiments,which shows the receptor’s rangeof sensitivity. Thresholds are conventionally described as the stimulus amplitude detected in half of the clinical trials. The measurement of sensory thresholds is a useful diagnostic technique for determining sensory function in individual modalities. Elevation of threshold may signal an abnormality in sensory receptors (such as loss of hair cells in the inner ear due to aging or exposure to loud noises), deficits in nerve conduction properties (as in multiple sclerosis), or a lesion in sensory processing areas of the brain. Sensory thresholds may also be affected as a result of emotional or psychological factors related to the conditions in which stimulus detection is measured. STIMULUS INTENSITY IS ENCODED BY THE FREQUENCY OF ACTION POTENTIALS IN SENSORY NERVES The details of neuronal activity; how long a neuron fires, how fast, and how many neurons are firing, encode the intensity and time course of sensory experience. In 1925 Edgar Adrian and Yngve Zotterman first noted that the discharge frequency of an afferent fibre increases with increasing stimulus intensity. This is because the activity of sensory receptors changes in relation to the stimulus amplitude. The change in membrane potential produced by the sensory stimulus is transformed into a digital pulse code, in which the frequency of action potentials reflects the amplitude of the receptor potential. Strong stimuli evoke larger receptor potentials,which generate a greater number and a higher frequency of action potentials. The timing of action potentials following depolarisation of a neuron depends on the neuron’s threshold for firing, which in turn varies depending on the neuron’s previous firing. Immediately after the action potential, an absolute refractory period of about 0.8 to 1.0ms means action potentials cannot be generated due to the inactivation of Na+ channels.The upper limiton neuronal firingis about1000 to 1200 spikes per second. The nerve fires a second impulse when the amplitude of the receptor potential exceeds the neuronal threshold. Receptor potentials of small amplitude are only slightly larger than the resting threshold. Therefore, the second impulse is generated late in the refractory period, resultingin a longinterval between the first and second spikes fired by the receptor’s axon. However, a large-amplitude receptor potential produced by a strong stimulus allows the threshold to be reached earlier in the refractory period, reducing the times between impulses. Thus, a large depolarisation produces a short interspike interval and high firing rates, whereas small depolarisation results in longinterspike intervals and low firing rates. In addition to increasing the frequency of firing, stronger stimuli also activate a greater number of receptors. These population codes contain receptors
- 4. 4 Coding of Sensory Information with low- and high-threshold receptors; when the stimulus intensity increases from weak to strong, low- threshold receptors are firstrecruited,followed by high-threshold receptors. THE DURATION OF A SENSATION IS DETERMINED IN PART BY THE ADAPTATION RATES OF RECEPTORS The temporal properties of a stimulus are encoded as changes in the frequency of sensory neuron activity. Stimuli appear, rise in intensity, fluctuate, or remain steady, and eventually disappear. Many receptors signal the rate at which the stimulus increases or decreases in intensity by rapidly changing their firing rate. For example, when a probe touches the skin, the initial spike discharge is proportional to the speed at which the skin is indented and the total amount of pressure (Figure 1.2A). During steady pressure the firing rate slows to a level proportional to skin indentation and firing stops when the probe is retracted. Thus, neurons signal important properties of stimuli notonly when they fire, but also when they stop firing. Figure 1.2 –Measurementsoffiring ratesquantify how sensory neuronsrepresent the intensity ofstimulation over time. In Figure 1.2A, asthe pressure increases, the total number ofaction potentialsdischarged rises, leading to higher firing rates. The firing rate is higher at the beginning of skin contact, as these receptors also detect how rapidly pressure is applied to the skin. In Figure 1.2B, rapidly adapting mechanoreceptorsonly respond at the beginning and end ofthe stimulus. The slope ofthe pressure pulse indicatesthe speed ofskin indentation in mm/s; all ofthe stimuli have the same final amplitude. Slowly applied pressure evokesalong-lasting burst oflow frequency firing; rapid indentation producesavery briefburst ofhigh frequency firing. Motion ofthe probe issignalled by the rate and duration of firing ofthis receptor. The receptor issilent when the indentation ismaintained at a fixed amplitude and fires again when the probe isremoved from theskin. Although the continuous firing of a sensory neuron encodes the intensity of the stimulus, if the stimulus persists for several minutes without a change in position or amplitude, its intensity diminishes and sensation is lost. This is called adaptation. All sensory receptors eventually adapt to constant stimulation, slowly adapting receptors are able to signal stimulus magnitude for several minutes. The stimulus duration is signalled by persistent depolarisation and generation of action potentials throughout the period of stimulation (Figure 1.2A), these receptors adapt gradually to a stimulus as a result of slow inactivation of Na + or Ca2+ channels by the depolarisingreceptor potential, or as a resultof activation of cal ciumdependent K+ channels.
- 5. Coding of Sensory Information 5 Some receptors cease firing in response to constant amplitude stimulation and are active only when the stimulus intensity increases or decreases. These rapidly adapting receptors respond only at the beginning and end of a stimulus, signalling the rate or velocity of stimulation (Figure 1.2B). Adaptation of rapidly adapting receptors depends on two factors. Firstly, in many of these receptors the prolonged depolarisation of the receptor potential inactivates the spike generation mechanism in the axon. Secondly, the receptor structure filters the steady components of the stimulus by changing shape, decreasing the electrical signal generated. The existence of two types of receptor is importantfor detecting contrasts in discretestimuli. Figure 1.3 – Receptor morphology influences adaptation in rapidly adapting mechanoreceptors. The Pacinian corpuscle isarapidly adapting mechanoreceptor located in the skin, in joint capsules, and in the mesentery ofthe abdominal wall. It consists of concentric, fluid-filled lamellae of connective tissue that form a capsule by surrounding the sensory nerve terminal. Because of this capsule, the sensory endings specialize in the detection of motion (lateral motion such as stroking, rubbing or palpation.) Figure 1.3A shows that when the capsule deflects steady pressure the receptor respondswith one or two action potentialsat the beginning and end ofapressure stimulus, but is silent when the stimulus is constant in intensity. When the stimulus first impinges on the skin, the capsule is deformed and compresses the nerve terminal. The pressure pulse activates stretch receptors in the nerve terminal, producing the response to stimulus onset. During steady pressure the capsule changes shape, reducing stretch of the nerve membrane. The outer lamellae of the capsule are compressed, absorbing the static load and preventing the deformation from being transmitted to the inner core of the capsule. When the pressure is removed, the capsule resumes its initial shape, and the resultant tissue movement stimulates the nerve terminal again, producing an “off” response. Figure 1.3B shows that Pacinian corpuscles are sensitive to vibration. Rapid movements are transmitted through the lamellae to the nerve terminal, generating areceptor potential and action potential for each vibratory cycle. SENSORY SYSTEMS PROCESS INFORMATION IN A SERIES OF RELAY NUCLEI The constituent pathways of sensory systems have a serial organization. Receptors project to first-order neurons in the CNS, which in turn project to second- and higher-order neurons. This sequence of connections gives rise to a distinct functional hierarchy. In the somatic sensory system, for example, primary afferent fibres converge onto second-order neurons, usually located in the CNS, and then onto third- and higher- neurons (Figure 1.3). The relay nuclei serve to pre-process sensory information and determine whether it is transmitted to the cortex. They filter out noise or sporadic activity (occurring at irregular intervals) in single fibres by transmitting only strong sequences of repetitive activity from individual sensory fibres or activity transmitted simultaneously by multiple receptors. The convergent connections from sensory receptors within the relay nucleus allow each of the higher-order neurons to interpret the sensory message in the context of activity in neighbouringinput channels. Like receptor neurons, neurons in each sensory relay nucleus have a receptive field. The receptive field of each relay neuron is defined by the population of pre-synaptic cells that converge on it. The receptive fields of second-order and higher-order sensory neurons are larger and more complex than those of receptor neurons. They are larger because they receive convergent input from many hundreds of receptors, each with a slightly different, but overlapping receptive field. They are more complex because they are sensitive to specific stimulus features,such as movement in a particular direction in thevisual field.
- 6. 6 Coding of Sensory Information Figure 1.4 – The functional and anatomical organization of sensory processing networks is hierarchical. Stimulation of a population of receptors initiates signals that are transmitted through a series of relay nuclei to higher centres in the brain (only one relay is shown here). At each processing stage the signalsare integrated into more complex sensory information. Figure 1.4A showseach relay neuron receiving sensory input from a large group of receptors, and thus obtaining a bigger receptive field than any of the neurons. The receptors closest to the stimulus respond more vigorously than the distant receptors. Figure 1.4B shows that with the addition of inhibitory interneurons (grey), the discharge zone is narrowed and on either side of the excitatory region the discharge rate is driven below the resting level by feedback inhibition. INHIBITORY INTERNEURONS WITHIN EACH RELAY NUCLEUS HELP SHARPEN CONTRAST BETWEEN STIMULI Inhibitory pathways are activated by three distinct pathways (Figure 1.5). The most important is where the afferent fibres of receptors or lower-order relay neurons make connections with inhibitory interneurons which have connections with nearby projection neurons in the nucleus. This feed-forward inhibition by afferent fibres allows the most active afferents to reduce the output of adjacent, less active projection neurons. It permits what Sherrington called a singleness of action, a winner-takes-all strategy, which ensures that only one of two or more competing responses is expressed. The inhibitory interneurons can also be activated by the projection neurons in the relay nucleus through recurrent axon collaterals from the projection neurons. This feedback inhibition allows the most active output neurons to limit the activity of the less active neurons. Such inhibitory networks create zones of contrasting activity within the CNS: a central zone of active neurons surrounded by a ringof less activeones. (Figure1.4B) Sensory information in the CNS is processed in stages, in the sequential relay nuclei of the spinal cord, brain stem, thalamus, and cerebral cortex. Each of these processing stations brings together sensory inputs from adjacent receptors and, using the works of inhibitory neurons, transforms the information to emphasise the strongest signals. (In the thalamus, prethalamic signals are translated so they can be processed by the cerebral cortex. The thalamus is believed to both process and relay sensory information selectively to various parts of the cerebral cortex, as one thalamic pointmay reach one or several regions in the cortex.) Stimulus Stimulus Receptors Relay neurons Relay neurons Spatialdistribution ofexcitationand inhibition Spatialdistribution ofexcitation
- 7. The Bodily Senses 7 Figure 1.5 – Inhibition of selected projection neurons in a sensory relay nucleus enhances the contrast between stimuli. The illustration shows three inhibitory pathways in the circuitry ofthe dorsal column nuclei, the first relay in the system for touch. The projection (or relay) cells (brown) send their axons to the thalamus. They receive excitatory input from touch receptor axons travelling in the dorsal columns. These afferent fibres also excite inhibitory interneurons (grey) that make feed-forward inhibitory connections onto adjacent projections cells. In addition, activity in the projection cells can inhibit surrounding cells by means of feedback connections. Finally, neurons in the cerebral cortex can modulate the firing ofprojection cellsby distal inhibition of either the terminals of primary sensory neuronsor the cell bodiesofprojectionneurons. THE BODILY SENSES Somatic sensibility has four major modalities: discriminative touch (required to recognise the size, shape, and texture of objects and their movement across the skin), proprioception (the sense of static position and movement of the limbs and the body), nociception (the signalling of tissue damage or chemical irritation, typically perceived as pain or itch), and temperature sense (warmth and cold). Each of these modalities is mediated by a distinct system of receptors and pathways to the brain, but all share a common class of sensory neurons; the dorsal root ganglion neurons. Individual dorsal root ganglion neurons respond selectively to specific types of stimuli becauseof morphological and molecular specialisation of their peripheral terminals. THE DORSAL ROOT GANGLION NEURON IS THE SENSORY RECEPTOR IN THE SOMATIC SENSORY SYSTEM All somatosensory information from the limbs and trunk is conveyed by dorsal root ganglia. Somatosensory information form cranial structures is transmitted by the trigeminal sensory neurons, which are functionally and morphologically homologous to dorsal root ganglion neurons. The dorsal root ganglion is well suited to its two principal functions of stimulus transduction and transmission of encoded stimulus information into the CNS. The cell body lies in a ganglion on the dorsal root of a spinal nerve, and its axon has two branches, one projecting to the periphery (where its specialised terminal is sensitive to a particular form of stimulus energy), and the other to the CNS. The terminal of the peripheral branch on the axon is the only portion of the dorsal root ganglion cell that is sensitive to natural stimuli. The properties of the nerve terminal determine the sensory function of each dorsal root ganglion neuron. The remainder of the peripheral branch, together with the central branch is called the primary afferent fibre; it transmits the encoded stimulus information to the spinal cord or brain stem. The peripheral terminals of dorsal root ganglion neurons can be either bare nerve endings, mediating thermal or painful sensations, or encapsulated by a non-neuronal structure (Figure 2.1), which mediate touch and proprioception, sensing stimuli that physically deform the receptive surface. Mechanoreceptors and proprioceptors are innervated by dorsal root ganglion neurons with large-diameter myelinated axons that conduct action potentials rapidly. Thermal receptors and nociceptors have small -diameter axons that are either unmyelinated or thinly myelinated, and conductimpulses more slowly. To Somatosensory Cortex
- 8. 8 The Bodily Senses Neurologists distinguish between two classes of somatic sensation: epicritic and protopathic. Epicritic sensations involve fine aspects of touch and are mediated by encapsulated receptors , they can detect gentle contact of the skin and localise the position that is touched (topognosis), discern the frequency and amplitude of vibration, resolve by touch spatial detail, and recognise the shape of objects grasped by the hand (stereognosis). Protopathic sensations involve pain, temperature, itch and tickle sensations and are mediated by receptors with bare nerve endings. Discriminating between these two types of sensation helps explain changes in sensation that followperipheral nerve damage. TOUCH IS MEDIATED BY MECHANORECEPTORS IN THE SKIN Figure 2.1 – The location and morphology of mechanoreceptors in hairy and hairless (glabrous) skin. Nerve fibresthat terminate in the superficial layersare branched at their distal terminalsinnervating several nearby receptor organs; nerve fibres in the subcutaneous layer innervate only a single receptor organ, whose structure determinesitsphysiologicalfunction. Tactile sensitivity is greatest on glabrous skin, which is characterised by a regular circular array of ridges formed by folds of the epidermis. These fingerprints contain a dense matrix of mechanoreceptors which mediate the sense of touch; they areexcited by indentation of the skin or motion across itssurface. The Meissner corpuscle is a rapidly-adapting receptor that is mechanically coupled to the ed ge of the papillary ridge, conferring fine mechanical sensitivity. The receptor is a globular, fluid filled structure that encloses a stack of flattened epithelial cells; the sensory nerve terminal is entwined between these various layers. The Merkel disk receptor, a slowly adapting receptor, is a small epithelial cell that surrounds the nerve terminal. It encloses a semi-rigid structure that transmits compressing strain from the skin to the sensory nerve ending, evoking sustained, slowly adapting responses. Merkel disk receptors are normally found in clusters at the centre of the papillary ridge. The Pacinian corpuscle is physiologically similar to the Meissner’s corpuscle. It detects rapid indentation of the skin and the large capsule of this receptor is flexibly attached to the skin, allowingit to sense vibration several centimetres away (Figure 1.3). These receptors are selectively activated by touching a tuning fork to the skin or a bony prominence. Ruffini endings are slowly adapting receptors that link the subcutaneous tissue to folds in the skin at the joints and in the palm or to the fingernails. These receptors sense stretch of the skin or the bending of the fingernails as these stimuli compress the nerve endings. Mechanical information sensed by the Ruffini endings contributes to our perception of the shapeof the grasped objects. Epidermal- dermal junction Bare nerve ending Meissner’s corpuscle Merkel disk receptor Hair receptor
- 9. The Bodily Senses 9 Similar mechanoreceptors are found in the hairy skin that covers most of the body surface. The principal rapidly adapting mechanoreceptors of the hairy skin are the hair follicle receptor and the field receptor. Hair follicle receptors respond to hair displacement and the three classes of receptors (down, guard and tylotrich hairs) differ in sensitivity to hair movement and conduction velocity. Field receptors are located primarily over the joints of the fingers, wristand elbow. They sense skin stretch when the jointis flexed or the skin is rubbed. Figure 2.2 –Mechanoreceptorsin glabrousskin vary in the size and structure oftheir receptive fields. Figure 1.2A showsthe Merkel disk receptor has a small, highly localised receptive field, whereas the Ruffini ending has a large field with a central zone of maximal sensitivity. Depending on their location, the Ruffini endings are excited by a stretch of the skin in specific directions (indicated by arrows). Figure 1.2B shows that Meissner’s corpuscles on the fingertips have receptive fields averaging 2-3mm Ø, where Meissner’s corpuscles on the palm have receptive fields averaging 10mm Ø. The receptive fields of Pacinian corpuscles cover larger continuous surfaces on the fingers or palm, with a central zone of normal sensitivity located directly above the receptor. Figure 2.2C shows expanded viewsofthe receptivefields; receptivefieldsin the superficial partsofthe skin have many pointsofhigh sensitivity. MECHANORECEPTORS IN THE SOMATOSENSORY AND DEEP LAYERS OF SKIN HAVE DIFFERENT RECEPTIVE FIELDS The size and structure of receptive fields differ for receptors in the superficial and deep layers of the skin. A single dorsal root ganglion innervating the superficial layers receives input from a cluster of 10-25 receptors. The afferent fibre has a receptive field that spans a small circular area of about 2-10mm Ø. In contrast, each nerve fibre innervating the deep layers innervates a single receptor. The fine spatial resolution in the fingertips allows humans to perform tactile discrimination of surface texture and read Braille, whereas the deep layers only resolvecoarsespatial differences froma wide area of skin.
- 10. 10 The Bodily Senses THE SPATIAL RESOLUTION OF STIMULI ON THE SKIN VARIES THROUGHOUT THE BODY BECAUSE THE DENSITY OF MECHANORECEPTORS VARIES The smallest receptive fields are found on the fingertips, they are slightly larger on the proximal phalanges and even bigger on the palm. The receptive fields in hairy skin also increase in area as stimuli are moved proximally from wrist to trunk. A sensory neuron innervating Meissner’s corpuscles and Merkel disk receptors transmits information about the largest skin indentation within its receptive field. The farther apart the points lie on the surface, the greater the likelihood that the two active nerves will be separated by silent nerve fibres. The contrast between active and inactive nerve fibres seems to be necessary for resolving spatial detail. The minimum distance between two detectable stimuli is called the two-point threshold, and varies for different body regions. Mechanoreceptors also differ in sensory thresholds; rapidly adapting receptors have much lower thresholds than slowly adapting receptors. The Pacinian corpuscle is the most sensitive, being able to detect the minute vibrations produced by impacts on a surface on which a hand rests or caused by the hum of an electric motor, or the frictional displacement of the skin when the hand moves across an object, regardless of whether the object is smooth or rough. The Meissner’s corpuscle is particularly sensitive to abrupt changes in the shape of objects that occur at the edges or corners and to small irregularities on the surface sensed during palpation, they are used to detect and localise small bumps or ridges on an otherwise smooth surface. More salient bumps are required to activate the slowly adapting Merkel disk receptors, but once activated they provide a clearer image of contours by changes in the frequency of firing. If the surface is flat these receptors fire continuously at relatively low rates. Convexities that indent the skin increase firing rates, while concavities silence these receptors. Large-diameter, gently curved objects also evoke weaker responses than small - diameters and the strongest responses occur when sharp edges contact the receptive field. Natural stimuli rarely activate a single type of receptor; rather they activate different combinations of mechanoreceptors that act synergistically. When we grasp, lift and replace an object on a surface, Meissner’s corpuscles are initially active as we grasp, then fire a second burst when the grip is released. Merkel disks are also initially active, but continue to fire until the grip is released. Pacinian receptors are most sensitive to transient mechanical pressures at the start and stop of the motion. The vertical gravitational forces applied to the skin as the object is lifted are signalled by Ruffini endings. SAI (Merkel) RA (Meissner’s) SAII (Ruffini) PC (Pacinian) Figure 2.3 –The distribution ofreceptor typesin the human hand varies. RA standsfor rapidly adapting, and SA for slowly adapting; RA and SA I are the most numerous and are preferentially distributed on the distal half of the fingertip. PC and SA II receptors are much lesscommon and are distributedmoreuniformly on thehand, showing littlevariation ofthedistal and proximal regions.
- 11. The Bodily Senses 11 Figure 2.4 –The firing patternsofmechanoreceptorsin the superficial layersofthe skin encode the texture ofobjectsrubbed acrossthe skin. The raised pattern on the drum moves horizontally across the immobilised monkey hand as the drum rotates. The experimente r thuscontrolsthe speed ofmovement and location ofthe dot pattern in the receptive field. The patter ismoved laterally on successive rotationsto allow the dotsto crossthe medial, central and lateral portionsofthe receptive field on successive rotations. The composite response ofan individual nerve fibre to successive viewsofraised dotssimulatesthe distribution ofactive and inactive nerve fibresin the population. Figure 2.4A2 shows each action potential represented as a dot, and each horizontal row of dots represents one scan with the pattern shifted laterally on the finger. Figure 2.4B shows that Meissner’s corpuscles differentiate between dots and blank space when the spacing of the dots exceeds the receptive field diameter. A receptor fires bursts of action potentials for each dot, spaced by silent intervals. Asthe dotsare broughtcloser together, the resolution blurs. OTHER SOMATIC SENSATIONS ARE MEDIATED BY A VARIETY OF SPECIALISED RECEPTORS WARMTH AND COLD ARE MEDIATED BY THERMAL RECEPTORS Warmth and cold are mediated by thermal receptors which detect the cold, cool, warm and hot; these thermal sensations arise from differences between the external temperature and normal body temperature of 34°C. At
- 12. 12 The Bodily Senses constant temperatures they have tonic discharges, firing action potentials at a constant rate governed by the actual temperature sensed. Unlike mechanoreceptors, thermoreceptors fire action potentials constantly at low rates (2-5 spike/second) when the skin temperature is set at its normal point of 34°C. The steady-state firing rate does not increase or decrease monotonically if the skin is slowly warmed or cooled. Instead, each class of thermal receptor shows peak firing at a preferred skin temperature. Cold receptors fire most vigorously at 25°C and warm receptors at 45°C. Individual warmth and cold receptors, therefore, do not give a precise reading of skin temperature, but involves comparing the relative activity of the different populations of thermal receptors and nociceptors. Rapid changes in skin temperature evoke dynamic responses, but if contact is maintained with the object for several seconds, the firing rate of the receptor drops to a lower rate. If the stimulus temperature exceeds 45°C, warmth receptors fire an intense burst of impulses and then cease firing even if the heat stimulus is maintained. Warmth receptors are unresponsive to hot temperatures, as stimuli above 50°C fail to excite them. At these high temperatures, humans perceive heat pain rather than sensations of warmth. PAIN IS MEDIATED BY NOCICEPTORS The receptors that respond selectively to stimuli that can damage tissue are called nociceptors (Latin nocere, to injure). They respond directly to some noxious stimuli and indirectly to others by means of one or more chemicals released from cells in the traumatised tissue. A variety of chemicals have been proposed to act as the chemical intermediary for pain in humans; histamine, K+, bradykinin, substance P and other related peptides, acidity, ATP, serotonin and acetylcholine. Because humans experience a burning pain when these nociceptors are stimulated, it is likely that they are chemoreceptors sensitive to the concentration of irritant chemicals released by the surrounding tissue by noxious thermal or mechanical stimuli, or to exogenous chemicals that may penetrate the skin and bind to their sensory endings. Some nociceptors respond to chemicals such as histamine, yielding itching sensations. As well as mechanical and thermal nociceptors, which are activated by particular forms of noxious stimuli, polymodal nociceptors, the largest class, are sensitive to the destructive effects of a stimulus rather than its physical properties. Figure 2.5 – Mechanical nociceptors are activated by strong stimuli and mediate sharp pricking sensations. Pressure on the cellsreceptive field with ablunt-tipped probe elicits no response even if the skin is indented by 2mm (A). But the tip of the needle that puncturesthe skin producesaclear response (B). Pinching the skin with serrated forceps ismore traumatic than apin prick and producesthestrongest response.
- 13. The Bodily Senses 13 Mechanical nociceptors require strong tactile stimuli, such as a pinch, in order to respond. They are also excited by sharp objects that penetrate, pinch or squeeze the skin (Figure 2.5). Their firing rates increase with the destructiveness of mechanical stimuli. The afferent fibres for mechanical nociceptors have bare nerve endings and arethe fastest conductingnociceptiveafferents becausethey are myel inated. Thermal nociceptors are excited by extremes of temperature as well as strong mechanical stimuli. One group is excited by noxious heat (>45°C), while a second responds to noxious cold (<5°C). Polymodal nociceptors are activated by noxious mechanical, heat and cold and chemical stimuli. These receptors are insensitive to gentle mechanical stimuli such as stroking the skin or light pressure. Stimulation of these receptors evokes sensations of slow, burning pain. Polymodal nociceptors provide the major sensory innervations of the tooth pulp. PROPRIOCEPTION IS MEDIATED BY MECHANORECEPTORS IN SKELETAL MUSCLE AND JOINT CAPSULES Proprioception (Latin proprius, belonging to oneself) is the movement and perception of one’s own limbs and body without the use of vision. It can be divided into limb-position sense (stationary position) and kinaesthesia (limb movement). These sensations are also important for manipulation of different objects and posture. Three types of mechanoreceptors in muscle and joints signal the stationary position of the limb and the speed and direction of movement. Muscle spindle receptors are specialised stretch receptors in muscle, Golgi tendon organs in tendons sense contractile force or effort exerted by a group of muscle fibres, and receptors in joint capsules sense flexion and extension of the joint. In addition, stretch-sensitive receptors in the skin (Ruffini endings, Merkel cells in hairy skin, and field receptors) also signal postural information. Cutaneous proprioception is particularly importantfor control of lip movements in speech and facial expression. THE VISCERA HAVE MECHANOSENSORY AND CHEMOSENSORY RECEPTORS The viscera are the internal organs, and sensory innervation plays an important role in the neural control of visceral function. (GI discomfort is mediated by receptors in the peritoneal lining of the gut). The viscera are innervated by dorsal root ganglion neurons with free nerve endings. The morphology of mechanosensory visceral afferents is similar to that of mechanical nociceptors in the skin. They are activated by distension and stretching of visceral muscle, which may evoke sensations of pain. Chemosensory nerve endings in the viscera play important roles in monitoring visceral function and provide the afferent limb for many autonomic reflexes. AFFERENT FIBRES CONVEYING DIFFERENT SOMATIC SENSORY MODALITIES HAVE DISTINCT TERMINAL PATTERNS IN THE SPINAL CORD AND MEDULLA The topographic arrangement of receptors in the skin is preserved as the central processes of the dorsal root ganglion neurons enter the spinal cord through the dorsal roots.The area of skin innervated by the nerve fibres comprisinga dorsal rootis called a dermatome. Dermatomes are arranged in a caudal-rostral sequence, the three branches of the trigeminal nerve (CN V) also preservethe topographic arrangements of receptors in the face through their projections to the trigeminal nuclei of the brain stem. Upon entry to the spinal cord thedorsal rootganglion neurons branch extensively and project to nuclei in the spinal grey matter and the brain stem. The spinal grey matter is divided into 10 laminae,each containing functionally distinctnuclei with different patterns of projections,and consists of the dorsal horn (LaminaeI-VI), the intermediate zone (Lamina VII), and the ventral horn (Laminae VIII and IX). Lamina X consists of the grey matter surroundingthe central canal.
- 14. 14 The Bodily Senses Figure 2.6 – Sensory information is conveyed to the thalamus and cerebral cortex by two ascending pathways. Tactile sensation and limb proprioception are transmitted to the thalamus ipsilaterally by the dorsal column-medial lemniscal system (alemniscusisa bunch of nerve fibres), while painful and thermal sensations are transmitted to the thalamus contralaterally by the anterolateral system. In the spinal cord the large-diameter dorsal root ganglion axons mediating touch and proprioception diverge from the smaller sensory afferentsfor pain and temperature sense. The large fibresascend in ipsilateral dorsal columnsto the brain where they terminate in the cuneate nucleus. The small fibres terminate on small-order neurons in the dorsal horn of the spinal cord, and the axons of these neuronscrossthe mid-line ofthe spinal cord to form the anterolateral tract. The second-order neuronsin the dorsal column nuclei send axons across the midline in the medulla, where they form the medial lemniscus. As these axons ascend through the brain stem they shift laterally, joining fibres of the spinothalmic tract in the midbrain before terminating in the ventral posterior lateral nucleus of the thalamus. Spinothalmic fibres terminate at nuclei not shown in this diagram. Thalamic neurons mediating touch and proprioception send their axons to the primary somatic sensory cortex in the post-central gyrus, while those sensitive to painful or thermal stimuli project to the primary somaticsensory cortex, thedorsal anterior insular cortex, and theanterior cingulate gyrus, rostral to thissection. Cerebral cortex Midbrain Pons Medulla Medulla Spinal cord
- 15. The Bodily Senses 15 The modalities of touch and proprioception are transmitted directly to the medulla through the ipsilateral dorsal columns (ipsilateral refers to something that is on the same side as another structure). Pain and temperature sense are relayed through synapses in the spinal cord to the contralateral anterolateral quadrant, where axons of dorsal horn neurons ascend to the brain stem and thalamus. The principal central branch of the axon of neurons mediating tactile sensation and proprioception from the limbs and trunk ascends in the ipsilateral dorsal columns to the medulla. Secondary branches terminate in the dorsal horn. Axons that enter the cord in the sacral region are found near the midline of the dorsal columns; axons that enter the cord at successively higher levels are added in progressively more lateral positions. At upper spinal levels the dorsal columns are divided into two bundles of axons: the gracile fasciscle (located medially, containing fibres from lower thoracic, lumbar and sacral vertebrae) and the cuneate fascicles (located laterally, containing fibres from the upper thoracic and cervical vertebrae). Mechanosensory information from the face and scalp is transmitted to the principal trigeminal nucleus, located in the pons, rostral to the dorsal column nuclei. The somatotropic organisation of the axons relayinginputfromreceptors in the skin and joints is maintained throughout the entire ascendingsomatosensory pathway,through the thalamus,to the somatosensory areas in the postcentral gyrus of the cerebral cortex. The axons of the neurons in the cuneate and gracilenuclei cross to the other sideof the brain stem and ascend to the ventral posterior lateral nucleus of the thalamus in the medial lemniscus. As the medial lemniscal fibres cross,thebody map becomes reversed, the sacral segments being located most laterally,and the cervical segments most medially. Because of the crossingof the fibres in the medulla and pons,the right sideof the brain receives sensory input from the limbs and trunk of the left sideof the body, and viceversa. The topographic arrangement of the axons changes as sensory information ascends in thebrainstem,so when the axons enter the thalamus they have the same mediolateral organisation asthe ventral posterior nuclei.Inputs from the legs are located most laterally,whilethosefrom the arm are located more medially. Although the dorsal columns contain both tactileand proprioceptiveaxons,these two submodalities remain anatomically segregated. The axons from proprioceptors arelocated more ventrally in the dorsal columns,and terminate more rostrally in thegracileand cuneate nuclei. THE ANTEROLATERAL SYSTEM MEDIATES SENSATIONS OF PAIN AND TEMPERATURE Neurons mediating sensations of pain or temperature from the limbs and trunk terminate in the ipsilateral dorsal horn of the spinal cord, and these smaller, unmyelinated axons branch extensively in the white matter forming the tract of Lissauer and terminate in the more superficial regions of the dorsal horn. Like the information on touch and proprioception conveyed in the dorsal column-medial lemniscal system, pain and temperature information also ascends to the thalamus in an anatomical pathway located in the anterolateral quadrant of the contralateral spinal cord (Figure 2.6). These spinal neurons send their axons across the midline of the spinal cord and ascend in the anterolateral column of the opposite sideof the body. Like axons in the dorsal columns, the axons of the anterolateral tract are arranged somatotopically (with a maintained spatial organisation within the CNS). Anterolateral fibres from sacral segments are located most laterally,whilethe cervical segments occupy the most medial locations. Unlike the medial lemniscus, which transmits sensory information directly to the thalamus, the anterolateral system has both direct and indirect paths to the thalamus. The anterolateral tract consists of three ascending pathways; the spinothalmic, spinoreticular and spinomesencephalic. The spinothalmic tract conveys information about painful and thermal stimuli to the thalamus; the spinoreticular tract relays information from the brain stem to the thalamus,and other structures such as the hypothalamus.
- 16. 16 Touch TOUCH Spatial properties are processed by populations of receptors that form many parallel pathways to the brain. It is the role of the CNS to construct a coherent image of an object from fragmented information conveyed in multiplepathways. THE PRIMARY SOMATIC SENSORY CORTEX INTEGRATES INFORMATION ABOUT TOUCH Sensory information is processed in a series of relay regions within the brain. Mechanoreceptors in the skin send their axons to the caudal medulla, where they terminate in the gracile or cuneate nuclei. These second- order neurons project directly to the contralateral thalamus, terminating in the ventral posterior lateral nucleus. A parallel pathway from the principal trigeminal nucleus, representing the face, ascends to the ventral posterior medial nucleus. The third-order neurons in the thalamus send axons to the primary somatic sensory cortex (S-I) located in the postcentral gyrus of the parietal lobe. The primary somatic cortex, SI, contai ns four cytoarchitectural ‘Brodmann’s areas’, 3a, 3b, 1 and 2 (Figure 3.1). Most thalamic fibres terminate in areas 3a and 3b, and the cells in these areas project their axons to areas 1 and 2. These four regions differ functionally; areas 3b and 1 receive information from receptors in the skin, whereas areas 3a and 2 receive proprioception from receptors in the muscle and joints. However, the four areas are extensively interconnected, so that both serial and parallel processingareinvolved in higher-order elaboration of sensory information. Figure 3.1 – The somatic sensory cortex has three major divisions: the primary and secondary somatosensory cortices (S-I and S- II) and the posterior parietal cortex (C). Figure 3.1A showsthe anatomical location of these three divisions. SI forms the most rostral portion of the parietal lobe; it covers the postcentral gyrus, beginning at the bottom of the central sulcus and extending posteriorly to the postcentral and intraparietal sulci. The postcentral gyrusalso extends into the medial wall of the hemisphere to the cingulate gyrus. The posterior parietal cortex (Brodmann’s areas 5-7) lies immediately posterior to S-I. S-II is located on the fissure ofStlvius. Figure 3.1B shows how the S-II cortex lies lateral to S-I and extends laterally, forming the superior bank ofthe lateral sulcus. Figure 3.1Cshows how S-I receives information from the ventral posterior lateral nucleus of the thalamus. Neurons in this area project mainly to areas 3b and 3b but also to 1 and 2. All these areas project to S-II and the posterior parietal cortex. Proprioceptive information from muscles andjoints Information from receptors in skin
- 17. Touch 17 The secondary somatic sensory cortex (S-II),located on the superior bank of the lateral fissure, is innervated by neurons from each of the areas of S-I (Figure 3.1C). These connections are required for the function of S-II. In contrast, removal of parts of S-II has no effect on the response of neurons in S-I. The S-II cortex projects to the insular cortex, which in term innervates regions of the temporal lobe believed to be important for tactile memory. Other important areas are Brodmann’s areas 5 and 7, which receive input from S-I and the pulvinar (the caudal-most nucleus of the thalamus) and this have an associational function. They are also bilaterally connected through the corpus callosum. Area 5 integrates tactile information from mechanoreceptors in the skin with proprioceptive inputs from the underlying muscles and joints. This region also integrates information from the two hands. Area 7 receives visual, tactile and proprioceptive inputs, allowing integration of stereognostic and visual information. The posterior parietal cortex projects to the frontal lobe and plays an important rolein sensory initiation and guidanceof movement. CORTICAL NEURONS ARE DEFINED BY THEIR RECEPTIVE FIELDS AS WELL AS BY MODALITY Like mechanoreceptors, the cortical neurons receiving sensory information from the skin are either slowly or rapidly adapting, signalling either the amplitude or rate of the peripheral skin indentation. Since each cortical neuron receives inputs from receptors in a specific area of the skin, central neurons also have receptive fields. Any point on the skin is represented in the cortex by a population of cortical cells connected to the afferent fibres that innervate that point on the skin. We perceive contact at a particular location on the skin bec ause a specific population of neurons in the brain is activated. When a point on the cortex is electrically stimulated, we also experience tactilesensations on a specificpartof the skin. The receptive fields of cortical neurons are much larger than those of dorsal root ganglions, the receptive cells on a finger cover tiny spots on the skin, while those of the cortical cells receiving these inputs are large areas covering an entire fingertip, or several adjacent fingers of the palmar surface of the contralateral hand (Figure 3.2). Receptive fields in higher cortical areas are even larger. In the posterior parietal cortex, receptive fields are often bilateral,located atsymmetric positions on the contralateral and ipsilateral hands. Figure 3.2 – The receptive fields of neurons in the primary somatic sensory cortex are larger than those ofthe sensory afferents. Each ofthe hand figurines show the receptive field of an individual neuron in areas 3b, 1, 2 and 5 of the primary somatic sensory cortex, based on recordings made in alert monkeys, where action potentials are elicited from the neuron from light touch to the coloured areas. Neurons that participate in later stages of neural processing (areas 1 and 2) have larger receptive fields and more specialised inputs than neurons in area3b. The neuron illustrated from area 2 is directionally sensitive to motion towards the fingertips. Neurons in area 5 often have symmetric bilateral receptive fields at mirror image locations on the contralateral and ipsilateral hand.
- 18. 18 Touch Cortical receptivefields encompass functional regions of skin thatareactivated simultaneously duringmotor activity.The sizeand position of cortical receptivefields on the skin arenot permanently fi xed,but can be modified through experience or injury to sensory nerves. Cortical receptive fields appear to be formed during development and maintained by simultaneous activation of the input pathways.Even though the receptive fields of cortical neurons cover a largearea of skin,it is still ableto discriminatefinedetail becauseit responds best to excitation in the middleof its receptive field.As the stimulation is moved to the periphery of the field, the signal becomes progressively weaker until eventually no spikes arerecorded. A stimulus to the tip of the index finger therefore strongly excites some neurons, whileothers fire weakly or not atall. THE PROPERTIES OF CORTICAL RECEPTIVE FIELDS ARE DUE TO CONVERGENT AND DIVERGENT CONNECTIONS IN THE RELAY NUCLEI The increase in area of the receptive fields of cortical neurons reflects the anatomical circuitry within the relay nuclei. Relay nuclei, such as the dorsal column, or thalamic nuclei, are composed of projection (or relay) neurons that send their axons to the next nucleus in the pathway and inhibitory interneurons that terminate upon relay neurons. Sensory inputs to the relay nucleus are characterised by extensive convergence and divergence. Ach sensory afferent has a branched terminal that innervates several postsynaptic neurons, so that each projection neuron receives synaptic input from many sensory neurons. This pattern of divergent presynaptic connections and convergent postsynaptic connections isrepeated at each relay in the pathway. INPUTS TO THE SOMATIC SENSORY CORTEX ARE ORGANISED IN COLUMNS BY RECEPTIVE FIELD AND MODALITY In a series of pioneering studies, Vernon Mountcastle discovered that the cortex is organised into vertical columns, 300-600 µm wide, spanning all six layers from the cortical surface to the white matter. All neurons within a column receive input from the same local area of skin and respond to a single class of receptors. Although the receptive fields comprising a column are not precisely congruent, they do share a common centre which is most apparent in layer IV (Figure 3.3C). A column therefore provides an anatomical structure that preserves the properties of location and modality. The columnar organisation of the cortex is a direct consequence of cortical circuitry. The pattern of intrinsic connections within the cerebral cortex is orientated vertically, perpendicular to the surface of the cortex. Thalamic afferents to the cortex terminate mainly in clusters of stellate cell neurons (neurons with several dendrites) in layer IV. The axons of the stellate cells project vertically towards the surface of the cortex (Figure 3.3A). In addition to sharing a common focal location on the skin, all of the neurons in a column usually respond to only one modality; touch, pressure, temperature or pain. Sensory receptors and primary sensory neurons responsive to one submodality, such as pressure or vibration, are connected to clusters of cells in the dorsal column nuclei and thalamus that receive inputs only for that submodality. These relay neurons in turn project to modality-specificcellsin thecortex. Although each of the four areas of S-I receive input from all areas of the body surface, one modality tends to dominate in each area. In 3a the dominant input is from proprioceptors sensing muscle stretch, area 3b receives input primarily from cutaneous mechanoreceptors. Here the inputs from a discrete site on the skin are divided into two sets of columns, one each for inputs from rapidly and slowly adapting receptors (Figure 3.4). In area 1, rapidly adapting cutaneous receptors predominate, and their receptive fields are considerably larger than those in area 3b, often covering several adjacent fingers. In area 2 and higher cortical areas, the modality segregation is much weaker. Columns of neurons in area 2 receive input from slowly and rapidly adapting cutaneous receptors and proprioceptors in the underlying muscles and joints. The receptive fields in areas 1 and 2 represent convergent input from regions of the hand and fingers thatare represented separately in areas 3a and 3b.
- 19. Touch 19 Figure 3.3 – The receptive field cellsin a column in Brodmann’s area 1 share a common central location on the skin. Figure 3.3A shows a sagittal section through the S-I cortex, illustrating the recording terminals of a group of neurons located on a single column. Figure 3.3A showsthe receptive fieldsof the four neurons shown in A. The neurons in this column share receptive fields on the ulnar portion of the forearm, wrist and hand. The dorsal and volar (palmar) surfaces of the hand and arm have been juxtaposed to illustrate the continuity of receptive fields along the ulnar margin. The receptive fields are labelled according to depth in the cortex. Figure 3.3C is a superimposition of all the receptor fields from Figure 3.3B, showing the associations of the different terminalson the neurons. Figure 3.4 –Each region ofthe somatic sensory cortex receivesinputs from primarily one type ofreceptor.Figure3.4A showsthat in each of the four regionsofthe somatic sensory cortex,inputsfrom one typeof receptor in specificpartsofthe body areorganisedin columnsof neuronsthat run from the surface to the white matter. Figure 3.4B showsdetail ofthecolumnar organisation ofinputsfrom digits2-5 in aportion ofBrodmann’sarea3b.Alternating columnsof neuronsreceive sensory inputfrom slowly adapting (SA)and rapidly adapting (RA)receptorsin thesuperficial layersofthe skin. Figure 3.4C showsthat overlapping receptivefieldsfrom RA and SA receptorsproject to distinctcolumnsofneuronsin area3b.
- 20. 20 Touch THE BODY SURFACE IS REPRESENTED IN THE BRAIN BY THE SOMATOTROPIC ARRANGEMENT OF SENSORY INPUTS The columns of neurons in the somatic sensory cortex are arranged such that there is a complete topographic representation of the body in each of the four Brodmann’s areas. The cortical map of the body corresponds to the spinal dermatomes defined by the afferent fibres entering the spinal cord at successively rostral layers . Sacral segments are represented medially, and cervical more laterally in the S-I cortex. The maps in adjacent cytoarchitectural areas are rough mirror images of the distal-proximal or dorsal-ventral axes of each dermatome (an area of skin that is supplied by a singlepair of dorsal roots). Topographic maps of the human cortex have been constructed from sensory-evoked potentials or by using electrical stimulation of the cortex. More modern, non-invasive tools include MEG (magnetoencephalography), fMRI (functional magnetic resonanceimaging) and PET (position emission tomography) INHIBITORY NETWORKS SHARPEN SPATIAL RESOLUTION BY RESTRICTING THE SPREAD OF EXCITATION For the purpose of somatotropic mapping, the receptive fields are identified by touching the skin with a small probe, but a more complex receptive field structure emerges when the skin is touched at two or more points. Stimulation of regions of skin surrounding the excitatory region of the receptive field of a cortical neuron may reduce the responsiveness of the neuron to an excitatory stimulus because afferent inputs surrounding the excitatory region are inhibitory.This spatial distribution serves to sharpen the peak of activity within the brain. The observed inhibitory responses in the cortex are generated by interneurons in the dorsal column nuclei, the ventral posterior lateral nucleus of the thalamus, and the cortex itself. Inhibitory interneurons in relay nuclei form circuits that tend to limit the spatial spread of excitation through divergent connections and not by inhibiting the peripheral receptors themselves. At the first relay point in the somatic sensory system, the afferent fibres inhibit the activity of cells in the dorsal column nuclei that surround the cells they excite (Figure 3.6). Inhibition generated by activity of the most intensively activated reduces the output of projection neurons that are less strongly excited. It permits a winner-takes-all strategy, ensuring the strongest of the competing responses is expressed. In addition the most active output neurons use recurrent collateral fibres to limit the activity of adjacent neurons. This lateral inhibition further sharpens the contrast between the active cells and their neighbours. Figure 3.5 – The somatotropic arrangement of somatosensory inputs in the human cortex is called a homunculus, where the image ofthe body in the brain exaggerates certain body regions, particularly the hand, foot and mouth, and compresses more proximal body parts. This map represents the innervation density of the skin, rather than its total surface area. In lower speciesthe hand representation in the brain issmaller than in primates, as these animals use other body parts, such as whiskers, to probe the environment. The representation of whisker fields in the cortex is larger than that of the paw. Neurons that receive projections from the whisker fields are arranged in discrete circular units called barrels. Each barrel is most responsive to asingle whisker. Imagesource: www.merck.com
- 21. Touch 21 LATERAL INHIBITION CAN AID IN TWO-POINT DISCRIMINATION We are able to distinguish two points rather than one because two distinct populations of neurons are activated. Stimuli applied to two widely spaced positions on the skin set up excitatory gradients of activity in two cell populations at every relay nucleus. If the two stimuli are brought close together, the activity in the two populations tends to overlap, and the distinction between the two peaks may become blurred. However, the inhibition produced by each stimulus also summates in the zone of overlap. As a result of this more effective inhibition,the peaks of activity in the two respondingpopulations become sharpened (Figure 3.7B). Merkel disk and Meissner’s corpuscles transmit a faithful neural image of embossed patterns. These sharp sensory images are preserved up to the firststage of cortical processing in area 3b of the S-I cortex. Neurons in area 3b fire bursts as each line segment of a letter is scanned across the receptive field and together faithfully signal its shape (similar idea to Figure 2.4A). The cortical representation of each letter is further sharpened by a pause in firing as the moving edges exit the excitatory receptive field and enter its inhibitory surround. Neurons in area 3b are able to signal the precise shape of the letters moved over the finger because their receptive fields are smaller than the letters. The individual line segments are viewed one at a time as they cross the neuron’s receptive field. The spatial arrangement of stimulated and unstimulated regions of skin is represented in the cortex in columns of active and silent neurons. In later stages of cortical processing, the responses are much more abstract, signalling only specific features common to groups of letters, such as vertical or horizontal lines.Itshould therefore be possibleto determine where the image becomes abstracted. Figure 3.6 – The receptive field of a higher-order neuron in the dorsal column nuclei has a characteristic pattern of excitation and inhibitionthat increasesspatial resolution. Figure 3.6A shows that many peripheral receptors converge onto a single second-order sensory neuron in the dorsal column nuclei. As a consequence, the excitatory receptive field of the central neuron is made up of the receptive fields of all the presynapticcells. Figure 3.6B shows that the receptive field of a neuron in the dorsal column nuclei and in the ventral posterior nuclei of the thalamustypically hasa central excitatory receptive field surrounded or flanked by an inhibitory region. The addition ofinhibitory interneurons(grey)narrowsthe discharge zone. Feed-forward inhibition sharpens the representation of a punctate stimulus by limiting the spread of excitation through convergent neural networks. On either side of the excitatory region the discharge rate is driven below the resting level by inhibition. Figure 3.6C shows that the asymmetric distribution of inhibitory interneurons produces lateral inhibition. In this schematic network, stimulation in the upper portion of the receptive field produces strong excitation of the relay neuron. Stimulation of the lower portion of the receptive field inhibits firing because the interneurons produce feed- forward inhibition. Stimulation in the zone of overlap of excitation and inhibition reduces the responsiveness of the relay neuron to the stimulus. Lateral inhibition isparticularly important for feature detection.
- 22. 22 Touch NEURONS IN HIGHER CORTICAL AREAS HAVE COMPLEX FEATURE-DETECTING PROPERTIES To produce a coherent sensation of an object, the nervous system must integrate information from a large number and variety of receptors as well as the modalities of touch, proprioception and temperature. At least four factors are involved: the size of the receptive field becomes larger at each level of processing (so the whole object can be detected), the profile of activity in the active population of neurons changes through the action of inhibitory networks, at successive levels of sensory processing in the cortex, individual neurons respond to more complex inputs, and the submodalities converge on individual neurons in association cortical areas. We have seen that neurons in area 3b of S-I provide a detailed representation of an individual object, while neurons in areas 1 and 2 are more concerned with abstract properties of tactile stimuli, signalling features such as motion, edge detection and the spatial arrangement of repeated patterns that form textures. Experiments using alert animals have revealed a variety of feature-detection neurons in the cortex. Some cortical neurons in area 2 respond preferentially to specific combinations of simultaneously stimulated receptors, some are orientation sensitive, sensing the angles of objects touched by the skin, or direction sensitive, some are even more specialised, sensing the spacing or alignment of ridges in a grating over the skin. The ability of a cortical neuron to detect the orientation of an edge or direction of motion results from the spatial arrangement of its neurons (Figure 3.8). The excitatory receptive fields are aligned along the preferred axis and produce a strong excitatory response when the stimulus orientation matches that of the receptive fields. In addition, the inhibitory receptive fields are placed to one side of the excitatory fields, suppressing inputs with the ‘wrong’ orientation or approachingfromthe ‘wrong’ direction. The projection of neurons from areas 3b and 3b onto areas 1 and 2 permit neurons ion area 2 to respond to complex features such as object shape, while neurons in area 1 only respond to touch, and neurons in area 3a to position sense, some neurons in area 2 have both inputs which respond best when an object of specific shape is grasped by hand. This information is thought to provide the necessary clues for skilled movement. Feature-detecting clues sensitive to stimulus orientation are first found in area 1,and then more extensively in area 2 (the areas concerned with stereognosis). Thus these complex stimulus properties arise from cortical processingof elementary inputs,and not from the thalamus. Figure 3.7 –Two point discrimination dependson separation ofthesignalsfrom each source.Figure3.7A showsstimulationofasingle point on the skin activating onepopulation ofcellsin thecortex.Maximalactivity isin the centreand thisissurrounded by aband of neuronswhose firing ratesare depressed below normal tonic levelsby the actionsofinterneuronsthat form lateralinhibitory networks. Figure 3.7B showshow stimulation oftwo adjacent peak pointsactivatestwo populationsofreceptors, each with apeak of activity (dotted lines). Normally the convergencewould resultin asingle largegroup ofexcitatory inputs, but lateral inhibitory networkssuppressexcitation oftheneuronsbetween thepoints, sharpening thecentral focusand preserving the spatial clarity ofthe original stimulus(solid line).
- 23. Touch 23 In the posterior parietal cortex (areas 5 and 7), the somatosensory responses are even more complex and often integrated with other sensory modalities, these association cortical areas play an important role in the sensory guidance of movement and are therefore organised functionally rather than topographically. Neurons in area 5 receive inputs from several adjacent groups of muscles that provide postural information, and neurons in area 7 integrate tactileand visual stimuli thatplay an importantrolein hand-eye co-ordination. Figure 3.8 –The spatial arrangement ofpresynaptic inputsto acortical neuron determined which specific featuresofa stimulus will activate the neuron. Motion from an inhibitory area to an excitatory area is less effective than the opposite direction because the long-lasting inhibitory postsynaptic potential evoked from the inhibitory field decreases the ability ofthe cell to respond when the stimulusmovesinto the excitatory field. Figure 3.8B demonstratesdirection sensitivity on acortical neuron. In thisexample, the preferred stimulusisahorizontal bar moving downward, be cause here, it crosses the excitatory fields of all three neurons simultaneously. Upward motion inhibits firing because it entersall three inhibitory fieldsfirst and the initial inhibition outlaststhe stimulus. STIMULUS FEATURES ARE PROCESSED IN PARALLEL BY DISTINCT AREAS OF CORTEX The somatosensory information necessary for stereognosis is processed in parallel in these areas because palpation involves repetitive touching of an object for several seconds. Such information is not simply relayed to different points in the brain; instead, tactile information being transmitted to higher cortical areas must be compared with recent information being processed at the early stages. Responses in areas 3a and 3b occur 20ms after touch or movement while the more posterior areas receive sensory information at longer latencies, processing stimuli presented 30-100ms earlier. Even though we don’t fully understand how the brain links all this information, studies indicate that various stimuli may be bound together by the brain through synchronised firingin differentcortical areas.
- 24. 24 The Perception of Pain Selective attention can modify firing patterns at the higher stages of cortical processing; neuronal responses in S-II depend on behavioural context or motivational state. The firing rates in a monkey can be altered by enforcing different patterns with rewards or by distracting the monkey with unrelated information. The same changes in circumstance have little effect on the spatial information conveyed by neurons in S-I. The S-II cortex provides the gateway to the temporal lobe via the insular cortex. Regions of the medial temporal lobe, particularly the hippocampus, are vital to the formation of memories and we only store information that has some behavioural significance. The demonstration that the firing patterns of S-II neurons are modified by selective attention suggests that S-II serves as a decision point for determining whether a particular bit of tactileinformation is remembered. LESIONS IN SOMATOSENSORY AREAS OF THE BRAIN PRODUCE SPECIFIC SENSORY DEFICITS The earliest information about the function of the somatic sensory system came from the analysis of disease states and traumatic injuries of the spinal cord. One of the late consequences of syphilitic infection in the nervous system is tabes dorsalis, which destroys the large-diameter neurons in the dorsal root ganglia, causing degeneration of myelinated afferent fibres in the dorsal columns. Patients with this degeneration have severe deficits in touch and position sense but often little loss of temperature sense and nociception. Additional information about the somatic afferent system has come from studies of behavioural defects produced by transaction of the dorsal columns of the spinal cord in experimental animals, or trauma in humans. Injury to the afferent somatosensory pathways of the dorsal columns results in a chronic deficit in certain tactile discriminations, such as detecting the direction of movement across the skin, the frequency of vibration, the relative position of two cutaneous stimuli and two-point discrimination. The deficit is ipsilateral to the lesion and occurs at levels below the lesion. After some extensive retraining and rehabilitation some information such as estimating the size of a probe pressed into the skin can be recovered, but perception of stimuli with complex temporal patterns is permanently impaired. In addition, lesions of the dorsal columns distort natural hand movements, such as control of fine finger movements during grooming, scratching and object manipulation in macaque monkeys. A similar, but reversible defect can be induced through pharmacological inhibition of neural activity of area 2 of the cortex. It has also been found through experimentation that total removal of S-I (areas 3a, 3b, 1 and 2) produces deficits in position sense and the ability to discriminate size, texture and shape. Thermal and pain sensibilities usually are not abolished,butaltered. In addition,serious motor deficits in hand function occur with major S-I lesions. Small lesions in area 3b produce deficits in the discrimination of object texture, size and shape. Lesions in area 1 produce a deficit in the assessment of texture, whereas lesions in area 2 alter the ability to differentiate size and shape. This is consistent with the idea that area 3b receives information about texture as well as size and shape. Area 3b also projects directly to areas 1 and 2. Because S-II receives inputs from all areas of S-I, excision causes severe impairment in the discrimination of shape and texture and prevents monkeys learning new tactile discriminations based on the shape of an object. Finally, damage to the posterior parietal cortex produces complex sensorimotor abnormalities, including the ability to accurately process stimuli in the contralateral visual field or the contralateral half of the body. Poor motor co-ordination and poor hand-eye co- ordination duringreaching,graspingand hand orientation lead to neglect in usageof the hand. THE PERCEPTION OF PAIN Pain is an unpleasant, sensory and emotional experience associated with actual or potential tissue damage. Nociception is the response to perceived or actual tissue damage, but does not necessarily lead to pain. Pain is highly individual and subjective and this makes it hard to define and treat clinically. Pain is not always immediately detected (such as soldiers in war) and chronic pains appear to serveno useful purpose.
- 25. The Perception of Pain 25 Persistent pain characterises many clinical conditions and encourages patients to seek medical attention. They can be sub-divided into nociceptive and neuropathic pains. Nociceptive pain can result from the direct activation of nociceptors in the skin or soft tissue in response to tissue injury, or in the case of sprains and strains. Neuropathic pains result from the direct injury to nerves in the peripheral or central NS and often have a burningor electric sensation. NOXIOUS INSULTS ACTIVATE NOCICEPTORS Thermal nociceptors are activated by extreme temperatures (>45°C or <5°C), and mechanical nociceptors are activated by intensive pressure applied to the skin. They both have small -diameter thinly myelinated Aδ fibres that conduct signals at about 5-30 m/s. Polymodal nociceptors are activated by high-intensity mechanical, chemical, or thermal stimuli. These nociceptors have small-diameter, non-myelinated C fibres that conduct slowly, generally at under 1.0 m/s. These three classes of nociceptors are widely distributed in skin and deep tissues and often work together. When you hit your hand with a hammer, for example, a sharp ‘first’ pain is felt immediately, followed later by a more prolongingaching/ burning‘second’ pain. The viscera contain silent nociceptors. Normally they are not activated by noxious stimulation, but their firing threshold is dramatically reduced by inflammation and by various chemical insults. Thus, the activation of silent nociceptors may contribute to the development of secondary hyperalgesia (excessive response to noxious stimuli at the site of injury and in surrounding undamaged tis sue) and central sensitisation (where nociceptive neurons in the dorsal horns of the spinal cord become sensitized by peripheral tissue damage or inflammation). Unlike the specialised receptors for touch and pressure, most nociceptors are free nerve endings and their mechanism of depolarisation is not known, although the nociceptor membrane is thought to contain proteins,like the capsaicin receptor (capsaicin is the activeingredient in hot peppers), that convert the noxious stimuli into a depolarising electrical potential. The vanilloid, or capsaicin, receptor is found exclusively in primary afferent nociceptors and mediates the pain-producing actions of capsaicin. Importantly, the receptor also responds to noxious heatstimuli,suggestingitis also a transducer of painful heatstimuli. Whereas the perception of touch or pressure is consistent when touch-pressure receptors are electrically stimulated, activation of the same nociceptor can lead to different reported sensations. This can be illustrated by placing a blood pressure cuff around a subject’s arm and inflated above systolic blood pressure for 30 minutes, producing temporary anoxia (a complete deprivation of oxygen supply) and blocks conduction in large-diameter Aα and Aβ fibres, while C fibres are still able to conduct action potentials and respond to noxious stimulation. This blockage occurs because Aα and Aβ fibres have a higher metabolic demand and, as a result, large motor neurons no longer conduct impulses causing the arm to be paralysed. In addition there is no touch, vibration, and the perception of pain is not normal as a pinch, a pin prick or an ice burn cannot be distinguished from each other, each of these distinct stimuli now all produce a slow burning pain. This experiment shows that large diameter Aβ fibres contribute to the normal perception of stimulus quality, even though they don’t respond directly to noxious stimuli. Although pain is unique, abnormal pain states can be reliably diagnosed, in pathological situations activation of nociceptors can lead to either allodynia or hyperalgesia. In Allodynia, pain results from stimuli that are normally innocuous, such as the light stroking of sunburnt skin. In contrast, patients with hyperalgesia, an excessiveresponseto noxious stimuli, often perceive pain spontaneously. NOCICEPTIVE AFFERENT FIBRES TERMINATE ON NEURONS IN THE DORSAL HORN OF THE SPINAL CORD The dorsal horn can be subdivided into six distinctlaminaeon the basis of cytological features (Figure4.1). Classes of primary afferentneurons conveying distinctmodalities terminatein distinctlaminae.
- 26. 26 The Perception of Pain Figure 4.1 – Nociceptive afferent fibres terminate on projection neurons in the dorsal horn of the spinal cord. Projection neurons in lamina I receive direct input from myelinated (Aδ) nociceptive afferent fibres and indirect input from unmyelinated (C) nociceptive fibres via stalk cell interneurons in lamina II. Lamina V neurons are predominantly of the wide dynamic-range type. They receive low- threshold input from the large-diameter myelinated fibres (Aβ) of mechanoreceptors as well as both direct and indirect input from nociceptive afferent fibres(Aδ and C). In thisfigure the lamina V neuron sends a dendrite up through laminaIV, where it iscontacted by the terminal of an Aβ primary afferent. A dendrite in lamina III arising from a cell in laminaV iscontacted by the axon terminal of alaminaII interneuron Nociceptive receptors are located in the superficial dorsal horn, in lamina I and II (the marginal area and substantia gelatinosa). The majority of these neurons receive direct input from Aδ and C fibres. Many of the neurons in the marginal area respond exclusively to noxious stimulation (nociceptive-specific neurons) and project to higher brain centres. Some neurons in this layer, called wide-dynamic-range neurons, respond in a graded fashion to both non-noxious and noxious mechanical stimuli. The substantia gelatinosa is made up almost exclusively of excitatory and inhibitory interneurons, some of which only respond to nociceptive inputs whileothers also respond to non-noxious stimuli. Laminae III and IV contain neurons that receive monosynaptic input from Aβ fibres. These neurons respond predominantly to non-noxious stimuli and have quite restricted receptive fields that are organised topographically. Lamina V contains primarily wide dynamic-range neurons that project to the brain and regions of the thalamus. These neurons receive monosynaptic input from Aβ and Aδ fibres (Figure 4.1). They also receive input from C fibres, either directly on their dendrites, which extend superficially into the dorsal horn, or indirectly via excitatory interneurons that receive input directly from C fibres. Many neurons in lamina V also receive nociceptive input from visceral structures. The convergence of somatic and visceral nociceptive input to lamina V neurons may explain ‘referred pain’; for example, myocardial infarction sufferers often report pain, not only from the chest, but also from the left arm. One explanation for this is that a single neuron receives input from both regions (Figure 4.2); as a consequence, higher centres cannot discriminate the source of input and incorrectly attribute the pain to the skin, possibly because cutaneous input normally predominates. An alternative basis for refereed pain is the branching of axons of periphery sensory neurons, but this is only likely in a minority of cases as single afferent fibres rarely innervate both a visceral and a remote cutaneous site. Neurons from lamina VI receive inputs from large-diameter afferents from muscles and joints, respond to non- noxious joint manipulation and are not thought to contribute to nociceptive transmission. Neurons in ventral horn laminae VII and VIII, many of which respond to noxious stimuli, have more complex response properties because the nociceptive inputs to lamina VII are polysynaptic. Also, although most dorsal horn neurons receive input from only one side of the body, many neurons in lamina VII respond to stimulation of either side and through brain stem connections may contribute to the diffuse(widespread) nature of many pain conditions.
- 27. The Perception of Pain 27 NOCICEPTIVE AFFERENT FIBRES USE GLUTAMATE AND NEUROPEPTIDES AS NEUROTRANSMITTERS The major excitatory neurotransmitter released by Aδ and C fibres and nociceptive afferents is the amino acid glutamate. The release of glutamate from sensory terminals evokes fast synaptic potentials in dorsal horn neurons by activating the AMPA-type glutamate receptors. The primary afferent fibres of nociceptive neurons also elicit slow excitatory postsynaptic potentials in dorsal horn neurons by releasing peptide transmitters. Small-diameter primary afferent terminals in the dorsal horn contain both small electron-translucent synaptic vesicles that store glutamate and large dense-core vesicles that store neuropeptides. Substance P is a neuropeptide that is released from C fibres in response to tissue injury or intense peripheral nerve stimulation. Glutamate and neuropeptides are released together from primary afferent terminals and have distinct physiological actions on postsynaptic neurons, but act co-ordinately to regulate the firing properties of post- synaptic neurons.Neuropeptides, includingsubstance P,appear to enhance and prolongglutamate’s actions. The range of action of the two classes of transmitters may also differ. The actions of glutamate released from sensory terminals are confined to postsynaptic neurons in the immediate vicinity of the synaptic terminal as a result of efficient uptake of amino acids into nerve terminals or glial cells (glial cells provide support and nutrition, maintain homeostasis, form myelin, and participate in signal transmission in the nervous system or nerve terminals). In contrast, neuropeptides released from sensory terminals can diffuse considerable distances because there is no specific reuptake mechanism. Thus, the release of neuropeptides from a single afferent fibre is likely to influence many postsynaptic dorsal horn neurons. This feature, with the fact that peptide levels are significantly increased in persistent pain conditions,suggests that peptide actions contribute both to the excitability of dorsal horn neurons and to the unlocalised character of many pa in conditions. HYPERALGESIA HAS BOTH PERIPHERAL AND CENTRAL ORIGINS CHANGES IN NOCICEPTOR SENSITIVITY UNDERLIE PRIMARY HYPERALGESIA Sensitisation of nociceptors can resultfromrepeated application of noxious mechanical stimuli (Figure4.3). Figure 4.2 – Signals from nociceptors in the viscera can be felt as pain elsewhere in the body. The source can be readily predicted from the siteofreferred pain. Figure 4.2A shows the sites of referred pain from myocardial infarction and angina. Figure 4.2B shows that convergence of visceral and somatic afferent fibres may account for the referred pain. In this hypothesis, nociceptive afferent fibres from the viscera and afferents from specific somatic areas of the periphery converge on the same projection neurons in the dorsal horn. The brain has no way of knowing the actual source of the noxious stimulus and mistakenly identifies the sensation with the peripheral structure.
- 28. 28 The Perception of Pain This mechanism is thought to be mediated by an axon reflex, similar to the spread of vasodilation in the vicinity of localised regions of cutaneous injury. The sensitisation of nociceptors after injury or inflammation results in the release of a variety of chemicals by the damaged cells and tissues in the vicinity of the injury, including bradykinin, histamine, prostaglandins, leukotrienes, ACh, serotonin and substance P (Table 4.1). Each originates from a different population of cells, but all act to decrease the thres hold for nociceptor activation. For example, histamine released from damaged mast cells in response to tissue injury activates polymodal nociceptors (Figure4.4). Table 4.1 –Naturally Occurring AgentsThat Activateor Sensitise Nociceptors Substance Source Enzyme involved in synthesis Effect on primary afferent fibres Potassium Damaged cells Activation Serotonin Platelets Tryptophan hydroxylase Activation Bradykinin Plasma kininogen Kallikrein Activation Histamine Mastcells Activation Prostaglandins Arachidonic acid –damaged cells Cyclooxygenase Sensitisation Leukotrienes Arachidonic acid –damaged cells 5-Lipoxygenase Sensitisation Substance P Primary afferents Sensitisation ATP, ACh, and serotonin are released from damaged endothelial cells and platelets and act alone or in combination to sensitise nociceptors via other chemical agents, such as prostaglandins and bradykinin. Prostaglandin E2 is a metabolite of arachidonic acid and is generated by the enzyme cyclooxygenase released from damaged cells. Aspirin and other non-steroidal anti-inflammatory analgesics (painkillers) are effective in controlling pain because they block cyclooxygenase, preventing prostaglandin synthesis. Bradykinin is one of the most active pain-producing agents; this high degree of activity is thought to result from its two distinct actions; activation of both Aδ and C nociceptors, and increase the synthesis and release of prostaglandins from nearby cells. Primary nociceptive neurons regulate their chemical environment through chemical modulators, which are synthesised in the cell body and then transported to the peripheral terminal, where they are stored and released upon depolarisation of the terminal. For example, injury leads to the release of substance P and calcitonin gene-related peptide from nociceptive sensory endings. These peptides contribute to the spread of oedema by directly dilating venules, and by leading to the release of histamine, which decreases the threshold for the activation of nociceptors. The main signs of inflammation are heat (calor), redness (rubor), and swelling (tumour). Local application of substance P can reproduce all of these symptoms. Calor and rubor result from dilation of peripheral blood vessels, whereas tumour results from plasma extravasation, where proteins and cells leak out of postcapillary venules, accompanied by fluid. Since this inflammation is mediated by neural activity, it is referred to as neurogenic inflammation. Non-peptide antagonists of substance P can completely block neurogenic inflammation in humans. THE HYPEREXCITABILITY OF DORSAL HORN NEURONS UNDERLIES CENTRALLY MEDIATED HYPERALGESIA Under conditions of severe and persistent injury, C fibres fire continuously and the response of the dorsal horn increases progressively. This phenomenon, called “wind up”, is dependent on the release of the excitatory transmitter glutamate from C fibres and consequent opening of postsynaptic ion channels gated by the N- methyl-D-aspartate (NMDA)-type glutamate receptor. Thus, blocking NMDA-type receptor activity can block wind up. Noxious stimulation can therefore produce long-term changes in dorsal horn neurons in a similar
- 29. The Perception of Pain 29 manner to long-term potentiation, by which long-term changes in synaptic transmission are elicited in the hippocampus and other regions of the brain. NMDA-type receptors also have a role in producing the hyperexcitability of dorsal neurons following tissue injury, or central sensitisation, to distinguish it from the sensitisation that occurs at the peripheral ending of sensory neurons via activation of the arachidonic acid cascade. These long-term changes in the excitability of dorsal horn neurons constitute a memory of the C fibre input in response to peripheral noxious stimuli; neurons in the dorsal horn show an induction of immediate early genes that encode transcription factors such as c-fos. There is also an upregulation in the expression of neuropeptides and neurotransmitters and their receptors that presumably changes the physiological properties of the neurons. Alterations in the biochemical properties and excitability of dorsal horn neurons can lead to spontaneous pain and can decrease the threshold for pain production. This is evident in the case of phantom limb pain. Until recently, limb amputation was performed under general anaesthesia in order to eliminate memory and awareness of the procedure. However. The spinal cord still ”experiences” the insult of the procedure since central sensitisation still occurs during a general. To prevent this central sensitisation, therefore, general anaesthesia is now supplemented with direct spinal administration of an analgesic or local infiltration of anaesthetics atthe injury site. Figure 4.3 – Thermal injury can sensitise nociceptors. Burns to the glabrous skin of the hand produce primary and secondary hyperalgesiato mechanical stimuli, but only primary hyperalgesiato heat stimuli. Mechanical thresholdsfor pain were recorded at sites A, B and C before and after burns at sites A and D. Figure 4.3A shows the sites of flare and mechanical hyperalgesia. In all subjects mechanical hyperalgesia was larger and remained present even after the flare disappeared. Figure 4.3B shows the mean mechanic al thresholdsfor pain before and after burnsin severalsubjects. The mechanical thresholdfor pain wassignificantly lower after theburn NOCICEPTIVE INFORMATION IS TRANSMITTED FROM THE SPINAL CORD TO THE THALAMUS AND CEREBRAL CORTEX ALONG FIVE ASCENDING PATHWAYS These pathways are the spinothalamic, spinoretucular, spinomesencephalic, cervicothalmic and spinohypothalmic tracts. The spinothalamic is the most prominent ascending nociceptive pathway in the spinal cord, it comprises the axons of nociceptive-specific and wide-dynamic-range neurons in laminae I and V-VII of the dorsal horn (Figure 4.5). These axons project to the contralateral side of the spinal cord and ascend in the anterolateral white matter, terminating in the thalamus. Electrical stimulation of this tract results in pain, whereas lesions of the tract result in marked reductions in pain sensation ipsilateral to the spinal cord lesion. The spinoretucular tract comprises the axons of neurons in laminae VII and VIII. It ascends to the anterolateral quadrant of the spinal cord and terminates in both the reticular formation and the thalamus. In contrast to the spinothalamic tract, many of the axons of the spinoretucular tract do not cross the midline. The spinomesencephalic tract comprises of the neurons in laminae I and V. It projects in the anterolateral quadrant
- 30. 30 The Perception of Pain of the spinal cord to the mesencephalic reticular formation and periaqueductal (or central) grey matter, and via the spinoparabrachial tract, it projects to the parabracihal nuclei and on to the amygdala, a major component of the limbic system (the neural system involved in emotion). Thus the spinomesencephalic tract is thought to contribute to the affective component of pain. Many of the axons in this pathway project to the dorsal part of the lateral funiculus, rather than the anterolateral quadrant. Thus, if these fibres are spared in surgical procedures designed to relieve pain,such as anterolateral cordotomy,pain may persistor recur. The cervicothalmic tract arises from neurons in the lateral cervical nucleus, located in the white matter of the upper two cervical segments of the spinal cord. The lateral cervical nucleus receives input from nociceptive neurons in laminae III and IV. Most of the axons in the cervicothalmic tract cross the midline and ascend in the medial lemniscus of the brain stem to nuclei in the mid-brain and to the ventroposterior lateral and posteromedial nuclei of the thalamus. Some axons from laminae III and IV project through the dorsal columns of the spinal cord (together with the axons of large-diameter myelinated primary afferent fibres) and terminate in the cuneate and gracile nuclei of the medulla. The spinohypothalmic tract comprises the axons of neurons in laminae I, V and VIII.It directly projects to the supraspinal autonomic control centres and is thought to activatecomplex neuroendochrine and cardiovascular responses. Figure 4.4 – Chemical mediators can sensitise and sometimes activate nociceptors. In jury or tissue damage releases bradykinin and prostaglandins, which activate or sensitise nociceptors leading to release of substance P and CGRP (calcitonin gene related peptide). Substance P acts on mast cells in the vicinity of sensory endings to evoke degranulation and histamine release, which directly excites nociceptors. Substance P causes plasma extravasion and GCRP dilates peripheral blood vessels. The resultant oedema causes additional bradykinin release THALAMIC NUCLEI RELAY AFFERENT INFORMATION TO THE CEREBRAL CORTEX Several nuclei in the thalamus process nociceptiveinformation.The lateral and medial nuclear groups are particularly important.The lateral nuclear group of the thalamus comprises the ventroposterior medial and lateral nuclei and the posterior nucleus.These nuclei receive input from the spinothalamictract,primarily from nociceptive-specific and wide-dynamic-changeneurons in laminaeI and V of the dorsal horn of the spinal cord. Neurons in these nuclei havesmall receptive fields,as do the spinal neurons thatprojectto them. The lateral thalamus may therefore be mostly concerned with mediating information aboutthe location of an injury,usually conveyed to consciousness asan acutepain.Injury to the spinothalamic tractand its targets causes a severe ‘central pain’. In addition,in certain chronic pain conditions,electrical stimulation of the thalamus results in intensepain,these observations emphasisethere is a change in thalamic and cortical circuits in chronic pain conditionsand patients who have experienced persistentpain due to injury have functionally differentbrains fromthose who have not experienced such pain. The medial nuclear group of the thalamus comprises the central lateral nucleus of the thalamus and the intralaminar complex.Its major inputis fromneurons in laminaeVII and VIII of the dorsal horn.The pathway to the medial thalamus is the firstspinothalamic projection to appear in mammalian evolution and is therefore known as the paleospinothalmic tract. It is also known as the spinoreticulothalamic tractas itincludes polysynaptic inputs viathereticular formation of the brain stem. The projection from the lateral thalamusto
- 31. The Perception of Pain 31 the ventroposterior lateral and medial nuclei isdeveloped in primates and i s therefore known as the neospinothalamic tract. Many neurons in the medial thalamus respond optimally to noxious stimuli butalso have widespread projections to the basal gangliaand many different cortical areas,and aretherefore also concerned with stimuli thatactivatea nonspecific arousal system. Figure 4.5 –Three ofthe major ascending pathwaysthat transmit nociceptive informationfrom thespinal cord to higher centres. THE CEREBRAL CORTEX CONTRIBUTES TO THE PROCESSING OF PAIN Neurons in several regions of the cerebral cortex respond to nociceptive input. Some of these neurons are located in the somatosensory cortex and have small receptive fields. Thus, they may not contribute to the diffuse aches that characterise most clinical pain. PET imaging studies also indicate that the cingulated gyrus and insular cortex are involved in nociception. The cingulated gyrus is part of the limbic system and is thought to be involved in processing the emotional component of pain. The insular cortex receives direct connections from the medial thalamic nuclei and from the ventral and medial thalamic nucleus, and processes information on the internal state of the body, contributing to the autonomic component of the overall pain response. Lesions of the insular cortex cause asymbolia for pain, where patients perceive noxious stimuli as painful, and can distinguish between sharp and dull pain, but do not show appropriate emotional responses. The insular cortex may therefore integrate the sensory, affective and cognitive components, all of which are necessary for normal responses. THE BALANCE OF ACTIVITY IN NOCICEPTIVE AND NON-NOCICEPTIVE PRIMARY AFFERENT FIBRES CAN MODULATE PAIN: THE GATE CONTROL THEORY Periaqueductal grey matter