Riboflavin,flavoproteins and their clinical applications
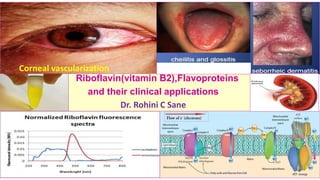
- 1. Riboflavin(vitamin B2),Flavoproteins and their clinical applications Dr. Rohini C Sane Corneal vascularization
- 2. ❖The word Riboflavin is derived from two sources : 1. Ribose (is a pentose sugar found in many biomolecules ).Ribitol is an open chain form of sugar ribose with aldehyde group (CHO) reduced to alcohol( CH2OH). 2. flavin (means yellow in Latin) ❖Warburg isolated the yellow enzyme of cellular respiration from yeast. (Noble prize 1931) ❖Paul Karrner (Noble prize 1937)-determined the structure of riboflavin.
- 3. Chemistry of Riboflavin • Riboflavin a) is a yellowish green compound . b) is 6,7dimethyl -9- D-ribitol - isoalloxazine which has a 6,7 dimethyl isoalloxazine ring (aheterocyclic3ringstructure) attached to D- ribitol (the reduced form of ribose) by a nitrogen atom. c) Molecular weight-C17H20N4O6 6,7dimethyl isoalloxazine ring D-ribitol
- 4. Antagonist of riboflavin • Dichlororiboflavin : by replacing two methyl (CH3)groups in riboflavin with chlorine atoms. • Isoriboflavin : by shifting methyl (CH3)groups in riboflavin to another positions. • Galactoflavin : antimetabolite of riboflavin
- 5. Properties of riboflavin (vitamin B2) ❖Properties of riboflavin (vitamin B2 ) : 1. Water soluble ( belongs to vitamin B complex) 2. Yellow orange colored 3. Functions as a Coenzymes (phosphorylated derivatives FMN,FAD) 4. Synthesized by gastrointestinal bacteria 5. Non toxic (limited gastric absorption→ no adverse effects with ingestion above RDA ) 6. Stable to heat in the neutral and acid medium 7. Destroyed in an alkaline medium even at room temperature and by improper cooking . 8. Aqueous solutions of riboflavin are unstable to visible and UV light → irreversible decomposition . It emits a strong greenish yellow fluorescence on exposure to UV light. ( therefore to be stored in amber colored glass bottles and property useful for its estimation) 9. Photosensitive = sensitive to light (on exposure to light , riboflavin decomposes to ribityl and lumichrome/ lumiflavin in acid / alkaline medium respectively) 10. Crystalizes in water at temperature < -30 C 11. can unite with metals like Fe and Mo thus forming metalloflavoproteins
- 6. Photodegradation of Riboflavin lumichrome(7,8 dimethyl isoalloxazine) in acid and neutral solutions Riboflavin UV light ribityl lumiflavin(7,8,10 trimethyl isoalloxazine- a yellow pigment soluble in chloroform and has greenish yellow fluorescence and formed by photodegradation of ribitol side chain of riboflavin at position 10 of isoalloxazine ring) in alkaline solutions →oxidation/ irreversible decomposition
- 7. Dietary sources of vitamin Riboflavin ❖Dietary sources of vitamin Riboflavin include : • Liver (Hepatoflavin*)-rich source • Eggs (Ovoflavin*) • Milk (Lactoflavin*) • Yeast • Green leafy vegetables • Cereals (fortification beneficial) • Fruits • Nuts • Mushroom * Structurally identical to riboflavin
- 8. Recommended Dietary allowances (RDA) of Riboflavin Category Recommended Dietary allowances (RDA) of Riboflavin Adults 1.3 -1.7 mg/day (19-70 years of age) Infants 0.7 mg/day Children 0.5 mg/day (up to age 8 ,increases progressively) Pregnancy 1.6 -2mg/day Lactation 1.6-2 mg/day(18-80 g of riboflavin secreted daily in human milk) Calculation of requirement and reference nutrient intake of Riboflavin have been based on protein allowances,energy intakes and metabolic body size.
- 9. ConditionsassociatedwithincreasedofDietary requirementof riboflavin (vitaminB2) Requirement of riboflavin(vitaminB2) increases during : 1.Pregnancy 2. Lactation 3.Increased calorie /protein intake 4.Convalescence 5.Acute illness 6. Severe injury 7.Infections 8. Severe burns 9.During oral broad spectrum antibiotic therapy
- 10. Biosynthesis of riboflavin • Human beings and animals cannot synthesize riboflavin and therefore dependent on dietary supply. In human beings ,considerable amounts can be synthesized by intestinal bacteria, but the quantity absorbed is not adequate to maintain its daily requirement. • Riboflavin content of seeds increases with germination .Geminating grams /dals are rich in riboflavin. • It is synthesized by yeast ,bacteria , fungi and all higher plants. • In nature, occurs as free form , nucleotide form and as flavoproteins.
- 11. Coenzyme forms of riboflavin ❖Riboflavin has two coenzymes. Both are nucleotides and integral part of enzymes. Such enzymes are called flavoproteins. 1. Flavin mononucleotide (FMN) : Flavin -ribityl-phosphate ( phosphate group attached to ribityl alcoholic group in position 5 ) 2. Flavin Adenine dinucleotide (FAD): Flavin-ribityl-phosphate-phosphate-ribose -Adenine (in FAD ,adenine nucleotide is attached to FMN by pyrophosphate linkage). FAD converted to form various tissue flavoproteins(mainly complexed with flavoprotein dehydrogenases and oxidases) and therefore, it is the predominant flavoenzyme present in tissue.
- 12. Structure of coenzymes FMN and FAD Isoalloxazine – ribitol – P P – ribose – Adenine riboflavin Adenosine Flavin mononucleotide Adenosine monophosphate (AMP) (FMN) Flavin adenine dinucleotide (FAD)
- 13. Reduction of FAD and FMN in oxidation process of the substrate During oxidation process, FAD and FMN accept two hydrogen atoms from the substrate .In turn, FAD and FMN are reduced to FADH2 and FMNH2 respectively. Two nitrogen atoms of isoalloxazine ring accept the hydrogen atoms in oxidation reactions . Acceptance of the hydrogen atoms by two nitrogen atoms of isoalloxazine ring
- 14. The functional unit of FAD is an isoalloxazine ring which serves as an acceptor of two hydrogen atoms (with electrons) to form FADH2. FADH2 undergo oxidation to form FAD( reversible reaction). FAD in oxidation reduction reactions (redox reactions)
- 15. Metabolic interconversion of riboflavin at cellular cytoplasm level ❖ FMN is synthesized from riboflavin by the enzyme flavokinase catalyzed phosphorylation. This reaction is reversed by FMN phosphatase .The FMN products can be complexed with specific apoenzymes to several functional flavoproteins but larger quantity is further converted to FAD. ❖ FAD is synthesized from FMN by enzyme FAD pyrophosphrylase/ FAD synthetase. This reaction is reversed by FAD pyrophosphatase. Cytoplasm of most tissue ,predominantly in small intestine ,liver ,heart , kidney + AMP ATP dependent ATP dependent
- 16. Metabolic interconversion of riboflavin • FAD converted to form various tissue flavoproteins (mainly complexed with flavoprotein dehydrogenases and oxidases) and therefore, it is predominant flavoenzyme present in tissue. • FAD can also become covalently linked to any of 5 amino acid residues of important apoenzymes. e.g. 8- N(3)-histidyl-FAD within succinate dehydrogenase, 8-s-cysteinyl-FAD within monoamine oxidase, both are mitochondrial in the location. • Turnover of covalently attached flavoenzymes require intracellular proteolysis and further degradation of the coenzymes involving nonspecific pyrophosphatase cleavage of FAD to FMN and AMP. This is followed action by nonspecific phosphatase on FMN and AMP.
- 18. Reductionof Flavinmononucleotide(FMN)andFlavinAdeninedinucleotide(FAD) The functional unit of both the coenzymes is an isoalloxazine ring which serves as an acceptor of two hydrogen atoms (with electrons) to form FMNH2 and FADH2.FMN and FAD undergo identical reversible reactions to form FMNH2 and FADH2.
- 19. Regulation of Metabolic interconversion of riboflavin • Biosynthesis of flavoenzymes , particularly at the flavokinase step is probably tightly regulated. • Thyroxine and triiodothyronine stimulate FAD and FMN biosynthesis in mammalian systems.
- 20. Functions of Riboflavin(vitamin B2)in the redox reactions • FMN and FAD are coenzymes and formed by ATP dependent phosphorylation of Riboflavin. • Riboflavin undergoes phosphorylation to form FMN by enzyme flavokinase in the small intestine . This reaction is a prerequisite for its absorption . FMN is then converted to FAD by enzyme ATP dependent FAD pyrophosphrylase / FAD synthetase in the liver. • The functional unit of both the coenzymes is an isoalloxazine ring which serves as an acceptor of two hydrogen atoms (with electrons) to form FMNH2 and FADH2. • FMNH2 and FADH2 undergo oxidation to form FMN and FAD(reversible reaction) respectively . • FMN and FAD take part in oxidation reduction reactions (redox reactions) responsible for energy production. • FMN and FAD are involved in the protection against peroxidation →involved in metabolism of xenobiotics in association with cytochrome P450.
- 21. Metabolism of riboflavin • Riboflavinisingestedintheformofflavoproteins.DuringdigestionFMN andFADcomponents arereleasedfromtheproteincomplexinthestomachasaconsequenceofgastricacidification andfreeriboflavinisreleasedintheintestine. • Absorptionofriboflavin:riboflavinis absorbedbythesaturable transportmechanismin proximalsmallintestine systemthatisrapidand proportionaltoitsintakebeforelevelingofat dosesnear27mg/day.Bilesaltsappeartofacilitateuptakeandamodestamountofvitamin circulatesviaenterohepaticsystem.Uptakeofriboflavinisindependentofsodiumions. • Transportofriboflavin inhumanbloodinvolveloosebindingtoalbuminandtightbinding to numberofglobulinswithmajorbeingtoseveral immunoglobulins(IgA,IgG,andIgM). • Theuptakeofriboflavinintothecellsoforganssuchasliver isfacilitatedpossiblyrequiringa specificcarrieratphysiologicalconcentrationbutcanbebydiffusionathigherconcentrations. Conversionof riboflavintocoenzymesoccurswithincellularcytoplasmofmosttissueparticularly in thesmallintestine,liver,heartand kidney.Riboflavinpresentinalltissueasnucleotidesbound toflavoproteins,higherconcentrationinliverand kidney. • Storageformofriboflavin foundinliverisFADandwhereitformscomplexesmainlywith numerousflavoproteindehydrogenasesandoxidases(itisverylittleinconcentration). • Urinaryexcretionofriboflavin:reflectdietaryintakeasonlysmallamountsofriboflavinisstored..
- 22. Metabolism of riboflavin in Pregnancyandlactation • Pregnancyincreasesthe concentrationof carrier proteinsfor riboflavin which resultin higher rate of riboflavin uptake at the maternal surfaceof the placenta. Pregnant women tend to excrete less riboflavin as pregnancyprogressesand additionallyexhibit FAD stimulationof erythrocyte reductaseactivity. • Milk containsreasonable quantitiesof the riboflavin and lesser quantities of coenzymes principallyFMN. During lactation,between 18 and 80g of riboflavin are secreteddaily into every 100 ml of human milk.
- 23. Flavoproteins • Flavoproteins :Enzymes that use flavin coenzymes (FMN or FAD) are called Flavoproteins. • Riboflavinpresentin all tissueasnucleotidesboundtoflavoproteins,higher concentrationin liver and kidney. • The coenzymes ( prosthetic groups) bind tightly to the protein (apoenzyme) either by non-covalent bond (mostly)or covalent bonds in the holoenzyme. • Many Flavoproteins contain metal atoms(iron, molybdenum etc.) and these are known as metalloflavoproteins .
- 24. Functions of Flavoproteins ❖Riboflavin(vitamin B2) is necessary for the maintenance of epithelial ,mucosal and ocular tissues. It is also called as a beauty vitamin. ❖Functions of Flavoproteins : 1. FAD and FMN linked Flavoproteins are associated with electron transport chain in mitochondria and certain enzymes involved in carbohydrate ,protein, lipid and purine metabolism. Thus FAD and FMN linked Flavoproteins being involved in energy production. 2. function as electrophiles and nucleophiles ,with covalent intermediates of flavin and substrate frequently being involved in catalysis . 3. catalyze dehydrogenation reactions , hydroxylation ,oxidative decarboxylation ,deoxygenation and reduction of oxygen to hydrogen peroxides. 4. function in drug metabolism in conjugation with the cytochrome P450. 5. are involved in the metabolism of iron, pyridoxine and folate. 6. have both prooxidative ( ability to produce superoxide and hydrogen peroxide contributing oxidative stress)and antioxidative function (FAD linked glutathione reductase involved in removal of lipid peroxides).
- 25. Enzymes dependent on coenzyme FMN and FAD FMN functions as a coenzyme for L- amino acid oxidases NADH dehydrogenase FAD functions as a coenzyme for Succinate dehydrogenase Pyruvate dehydrogenase complex Alpha ketoglutarate dehydrogenase complex Acyl Co dehydrogenase Xanthine oxidase D- amino acid oxidases
- 26. FAD and FMN are associated with electron transport chain located in the inner mitochondrial membrane NADH dehydrogenase is a flavoprotein enzyme associated with respiratory chain / electron transport chain (located in the inner mitochondrial membrane) and responsible for energy production. v
- 27. Flavoprotein NADH dehydrogenase ( NADH coenzyme Q reductase ) is a component of the electron transport chain in the inner mitochondrial membrane and has FMN as the prosthetic group which accepts two elections and protons to form FMNH2 . : NADH + H+ + FMN → NAD + FMNH2 Function of FMN in NADH dehydrogenase (NADH coenzyme Q reductase ) of electron transport chain in the inner mitochondrial membrane involved in energy production
- 28. Flavoprotein Succinate dehydrogenase (Succinate coenzyme Q reductase) is an enzyme found in the inner mitochondrial membrane and needs FAD as the coenzyme. FAD accepts two hydrogen atoms ( 2 H+ + 2 e - )from succinate to form fumarate. Succinate + FAD → fumarate + FADH2 Function of FAD in Succinate dehydrogenase (Succinate coenzyme Q reductase) of the electron transport chain located in the inner mitochondrial membrane Function of FAD in Succinate dehydrogenase involved in energy production
- 29. Function of FMNH2 andFADH2 aselectron donor for Coenzyme Q in the electron transport chain located in the inner mitochondrial membrane Coenzyme Q(ubiquinone) is a lipophilic electron carrier. It can accept electrons from FMNH2 produced in the electron transport chain (ETC) by NADH dehydrogenase or FADH2 produced outside ETC ( Succinate dehydrogenase, Acyl CoA dehydrogenase). FMNH2 / FADH2 are then oxidized to FMN /FAD respectively to continue ETC for energy production via TCA / beta oxidation of fatty acids.
- 30. FAD is associated with mitochondrial Glycerol 3- phosphate dehydrogenase shuttle The inner mitochondrial membrane is impermeable to NADH .Cytosolic NADH are transported to mitochondria by glycerol 3- phosphate dehydrogenase shuttle . Cytosolic glycerol 3- phosphate hydrogenase oxidizes NADH to NAD + . The cytosolic reducing equivalents are transported through glycerol 3- phosphate into the mitochondria . Glycerol 3- phosphate dehydrogenase present on the outer surface of inner mitochondrial membrane reduces FAD to FADH2 . FADH2 gets oxidized via CoQ of ETC (togenerate1.5ATP). Dihydroxy acetone phosphate escapes into cytosol and the shuttling continues as depicted.
- 31. Flavoprotein enzymes and their reactions Pathway/ Metabolism Flavoprotein enzymes Reactions Carbohydrate Metabolism Mitochondrial Glycerol- 3- phosphate dehydrogenase shuttle the shuttling of reducing equivalents from cytosol to mitochondria (ETC) TCA /Citric acid cycle Pyruvate dehydrogenase complex Dihydrolipoyl dehydrogenase component requires FAD as a coenzyme ( Pyruvate →Acetyl CoA+CO2) oxidative decarboxylation Alpha Ketoglutarate dehydrogenase complex ( Alpha Ketoglutarate→ Succinyl CoA+ CO2 ) →oxidative decarboxylation Succinate dehydrogenase (Succinate →Fumarate ) Protein Metabolism Glycine Oxidase ( Glycine→ Glyoxylate +NH3) D- amino oxidases (D- Amino Acid → Keto Acid + NH3) L- Amino Oxidases (FMN dependent enzyme) (L- Amino Acid → Keto Acid + NH3) Deamination of amino acids Lipid Metabolism Fatty Acyl CoA dehydrogenase (Acyl CoA→ β unsaturated Acyl CoA) Fatty acid oxidation Purine Metabolism Xanthine oxidase (Xanthine → Uric acid) FAD dependent enzymes/ Flavoproteins
- 32. Pyruvate Dehydrogenase Complex ❖ Pyruvate Dehydrogenase Complex has three enzymes : 1. Pyruvate Dehydrogenase 2. Dihydrolipoyl Dehydrogenase(diaphorase) 3. Dihydrolipoyl Transacetylase ❖ Pyruvate Dehydrogenase Complex has five coenzymes : 1) TPP 2) FAD 3) NAD+ 4) CoASH 5) Lipoic acid ❖Pyruvate Dehydrogenase Complex uses Magnesium (Mg 2+) as a cofactor.
- 33. Biochemical Functions of riboflavin: oxidative decarboxylation of Pyruvate for synthesis of Acetyl CoA Pyruvate Dehydrogenase catalyzes oxidative decarboxylation of Pyruvate to Acetyl CoA (used in TCA) and Carbon dioxide. Dihydrolipoyl dehydrogenase component requires FAD. Coenzyme role of FAD in Pyruvate Dehydrogenase Complex
- 34. OxidativedecarboxylationbyFADlinkedPyruvatedehydrogenasecomplex (PDHcomplex)isneededforbiosynthesisofbiomolecules ❖ oxidative decarboxylation of Pyruvate →Acetyl CoA + CO2 • Acetyl CoA is involved energy production via TCA and in the biosynthesis of Cholesterol, fatty acids, ketone bodies, Acetyl choline ,Glycoproteins, Urea ,N- Acetyl neuraminic acid (ganglioside) . It also plays role in xenobiotic metabolism.
- 35. Alpha ketoglutarate dehydrogenase complex ❖ Alpha ketoglutarate dehydrogenase complex has three enzymes : 1. Alpha ketoglutarate dehydrogenase 2. Dihydrolipoyl Dehydrogenase 3. Dihydrolipoyl Trans-succinylase ❖ Alpha ketoglutarate Complex has five coenzymes : 1) TPP 2) FAD 3) NAD+ 4) CoASH 5) Lipoic acid
- 36. Coenzyme role of FAD in Alpha ketoglutarate dehydrogenase complex for synthesis of Succinyl CoA Alpha ketoglutarate dehydrogenase complex catalyzes oxidative decarboxylation of Alpha ketoglutarate to Succinyl CoA and Carbon dioxide (in TCA). Dihydrolipoyl dehydrogenase component requires FAD. Succinyl CoA is metabolized to Fumarate in TCA or Aminolaevulinic acid( heme synthesis). Biochemical Functions of riboflavin in oxidative decarboxylation of Alpha ketoglutarate to Succinyl CoA FAD
- 37. Role of FAD linked Succinate dehydrogenase in TCA /Citric acid cycle Succinate is dehydrogenated by FAD linked Succinate dehydrogenase to Fumarate. FAD is reduced to FADH2 in this reaction.FADH2 then enters in electron transport chain in the inner mitochondrial membrane to generate 1.5 ATP. Succinate dehydrogenase is an enzyme found in the inner mitochondrial membrane.
- 38. Role of FMN and FAD in oxidation of amino acid (L or D) L – Amino acid oxidase and D –amino acid oxidase contain FMN and FAD as coenzyme respectively. They act on the corresponding amino acids (L or D) to produce ketoacids and NH3 during oxidative deamination of amino acid (L or D). In this reaction ,oxygen is reduced to H2O2 which later decomposed by catalase to H2O. D –amino acid present in diet are metabolized by the liver using this pathway. Role of FMN and FAD in oxidative deamination of amino acid (L or D) Amino acid oxidases use auto oxidable flavins which oxidizes amino acid to -imino acid. -imino acid decomposes to ketoacids . Ketoacids →TCA , Gluconeogenesis
- 39. Alpha Keto Acid Dehydrogenase complex involved in oxidation of branched chain amino acids :1 (uses coenzymes –TPP , NAD+, Lipoic acid and Mg 2 +as a cofactor ) Biochemical Functions of riboflavin in oxidation of branched chain amino acids
- 40. FAD linked Oxidation of branched chain amino acids Valine Leucine Isoleucine Transamination branched chain amino acid transaminase ketoisovalerate ketoisocaproate keto - -methyl valerate keto acids Oxidative Decarboxylation → CO2 → CO2 → CO2 Isobutyryl CoA Isovaleryl CoA methyl butyryl CoA ,unsaturated acyl CoA thioesters CO2 → Biotin Methylacrylyl CoA Methyl Crotonyl CoA Tigyl CoA FAD FADlinked Acyl dehydrogenase complex FADH2 Propionyl CoA HMG CoA Methyl acetoacetyl CoA Biotin, CO2→ CO2 Lyase → Acetyl CoA→ Fat → Acetyl CoA → Fat Methyl malonyl CoA Acetoacetate Propionyl CoA → Glucose Heme Vitamin B12→ Biotin ,CO2→ Vitamin B12 Heme Succinyl CoA → Glucose Fat Methyl malonyl CoA → Succinyl CoA → Glucose Keto Acid Dehydrogenase complex ,TPP, NAD+ , CoASH HMG CoA is a precursor for cholesterol biosynthesis and ketone body formation.
- 41. ❖Branched chain alpha keto acids of Valine ,Leucine, Isoleucine are : ketoisovalerate, ketoisocaproate , keto - -methyl valerate ( corresponding ketoacids of Valine ,Leucine, Isoleucine ) + Keto Acid Dehydrogenase complex (uses coenzymes –TPP, NAD+, FAD , Lipoic acid and Mg 2 +as a cofactor ) Transfer of activated CHO group to Alpha Lipoic Acid Isobutyryl CoA , isovaleryl CoA , methyl butyryl CoA → synthesis of Acetyl CoA ( corresponding , unsaturated acyl CoA thioesters) or succinyl CoA Coenzyme role of FAD in Alpha Keto Acid Dehydrogenase complex of branched chain amino acids in oxidation of their alpha keto acids :2 Alpha Keto Acid Dehydrogenase catalyzes oxidative decarboxylation of ketoacids of branched chain amino acids to , unsaturated acyl CoA thioesters and carbon dioxide .FAD functions as a coenzyme in this reaction.
- 42. Fate of branched chain amino acids unsaturated acyl CoA thioesters undergo oxidation by enzymes Acyl CoA Dehydrogenase complex ( 2 enzymes )which uses FAD as a coenzyme. End products oxidation of branched chain amino acids are Acetyl CoA , Acetoacetic acids or Succinyl CoA which are needed for biosynthesis of many biomolecules . FAD FAD FAD
- 43. Role of FAD in Beta Oxidation of Fatty Acids in mitochondria :1 Oxidation Oxidation Hydration Cleavage Each cycle of Beta Oxidation of Fatty Acids, liberating Acetyl CoA ,occurs in sequence of four reactions : Oxidation , Hydration, Oxidation and Cleavage . The overall reaction for each cycle of oxidation : C n Acyl CoA + FAD + NAD+ + H 2O + CoASH →C(n-2)+ Acetyl CoA + FADH2+NADH +H+ Each of FADH2 and NADH generated in beta oxidation of fatty acids are oxidized in electron transport chain- ETC to synthesize ATP(1.5 and 2.5 respectively ).
- 44. Role of FAD in Beta Oxidation of Fatty Acids in the mitochondria :2 In the first reaction of beta oxidation of fatty acids in the mitochondria : Acyl CoA undergoes dehydrogenation by a FAD linked flavoenzyme Acyl CoA dehydrogenase. A double bond is formed between and carbons ( 2 and 3 carbons ) to form trans-2- Enoyl- CoA.
- 45. Role of FMN in Beta Oxidation of Fatty Acids:3 Each FADH2 AND NADH generated in beta oxidation of fatty acids are oxidized in electron transport chain- ETC to synthesize ATP(1.5 and 2.5 respectively ) . hydroxy acyl CoA is produced in second step of beta oxidation fatty acid and it is oxidized by NADH linked Beta hydroxy acyl CoA dehydrogenase. Reducing equivalents of NADH are transported in ETC via FMN of NADH dehydrogenase of mitochondria to synthesize 2.5 ATP.
- 46. Function of FAD as a prooxidant in Beta Oxidation of long chain Fatty Acids in peroxisomes • Peroxisomes (organelles present in most eukaryotes)carry out initial oxidation of long chain fatty acids (C20,C22 etc.) which is followed by mitochondrial oxidation. • Beta oxidation of long chain fatty acids is catalyzed by FAD linked acyl CoA dehydrogenase leading to formation of FADH2. Reducing equivalents of FADH2 are not transferred to electron transport chain ,but handed directly to O2 leading to formation of H2O2 ,which is then cleaved by Catalase . E-FADH2 + O2→ E-FAD + H2O2 (FAD functions as a prooxidant inducing oxidative stress ) Catalase H2O2 H2O + ½ O2 Therefore is no ATP synthesis in Peroxisomal beta oxidation of long chain fatty acids however heat is liberated. ✓Peroxisomal beta oxidation is induced by high fat diet and administration of hypolipemic drugs ( e.g. Clofibrate).
- 47. NeuroendocrinefunctionofFADlinkedMAOincatabolismofCatecholamines ❖ Catecholamines : are derivatives of tyrosine and contain Catechol ring (dihydroxy benzene ) .They are compounds that consist of monoamines. ❖The principle Catecholamines are : 1. Adrenaline /epinephrine →secondary monoamine 2. Noradrenaline/norepinephrine primary monoamines 3. Dopamine ✓The difference between Dopamine and Noradrenaline : is one additional hydroxy group in the structure of Noradrenaline . ✓The difference between Adrenaline and Noradrenaline is only a methyl group . Noradrenaline has no methyl group and its Methylation by S-adenosyl methionine-SAM yields Adrenaline . ❖All three catecholamines need FAD linked Monoamine oxidase (MAO) enzyme for their catabolism by oxidative deamination to VMA (Adrenaline, Noradrenaline) or homovallinic acid (Dopamine)which are later excreted in urine.
- 48. Structure of Tyrosine , Catechol ,Dopamine , Noradrenaline and Adrenaline (Dihydroxy benzene ring) Catechol Primary monoamines Secondary monoamine
- 49. Functions of Adrenaline ❖Catecholamines (most predominantly Adrenaline) increase 1. Cardiac output by increasing the rate and force of myocardial contraction 2. Blood pressure 3. Oxygen consumption 4. Glycogenolysis in liver and muscles contributing hyperglycemia 5. Gluconeogenesis (anti-insulin in nature) 6. Lipolysis (anti-insulin in nature)releasing free fatty acids from adipose tissue for hepatic gluconeogenesis 7. action via the - adrenergic receptors → increases secretion of Glucagon , Thyroxine , Calcitonin ,Renin , Gastrin 8. Relaxation of smooth muscles of bronchiole tree of lung (useful in treatment of asthma), gastrointestinal tract ,gall bladder ❖Released from adrenal medulla in response fight, fright, exercise, and hypoglycemia( half-life of Adrenaline is 2-5 minutes) ❖Adrenaline inhibits insulin secretion from the pancreas by acting via adrenergic receptors .
- 50. Functions of Noradrenaline ❖Functions of Noradrenaline : 1. is primarily synthesized in sympathetic nervous system and acts locally as a neurotransmitter in the postsynaptic cell. 2. has vasoconstrictor effect and hence increase blood pressure (both systolic and diastolic). 3. is useful in the treatment of hypotensive shock other than caused by hemorrhage due to its marked action on the arterioles without producing tachycardia.
- 51. Biosynthesis of Adrenaline and Noradrenaline • Adrenaline is primarily synthesized and stored(80%) in adrenal medulla. • Noradrenaline is primarily synthesized in sympathetic nervous system and acts locally as a neurotransmitter at the postsynaptic cell. It is also synthesized and stored (20%) in adrenal medulla. • Adrenaline and Noradrenaline are produced from Phenylalanine and Tyrosine. • In pheochromocytes and neuronal cells of adrenal gland, the synthesis of catecholamines is essentially same. Both are stored in the form of granules . • Noradrenaline only occurs in adrenergic nerve terminals as granules or as vesicles as a complex containing ATP ( ratio 4 : 1) and in combination with proteins chromogenin A and chromoembrin B.
- 52. Metabolism of Adrenaline and Noradrenaline Liver : Phenylalanine → Tyrosine catalyzed by Phenylalanine hydroxylase ,O2 ,NADPH, Tetrahydrobiopterin Tyrosine enters mitochondria Tyrosine hydroxylase O2 ,NADPH ,Tetrahydrobiopterin Mitochondria DOPA (Dihydroxy Phenylalanine) enters cytoplasm DOPA decarboxylase PLP Cytosol CO2 Dopamine enters chromaffin granules Granulated vesicles of brain cells Dopamine hydroxylase Cu2+, Vit C vesicles or granules of pheochromocytes or nerve endings Noradrenaline enters cytoplasm N –Methyl transferase SAM Cytosol in nerve cells synthesis ends, noradrenaline stored in SAH granulated vesicles Adrenaline Catechol-O-methyl transferase (COMT) SAM Cytosol of liver or target organs of after their synthesis in pheochromocytes→ transported to SAH chromaffin granules for storage Metanephrine FAD linked monoamine oxidase (MAO) mitochondria of liver or target organs of Vanillyl mandelic acid (VMA)→ nerve terminus→ urine Dopamine, Noradrenaline , Adrenaline, Metaepinephrine and Vanillyl mandelic acid are synthesized in adrenal medulla and sympathetic ganglia.
- 53. Function of FAD linked Monoamine oxidase (MAO) in Metabolic degradation of catecholamines in liver and target organs Liver : Phenylalanine → Tyrosine catalyzed by phenylalanine hydroxylase ,O2,NADPH,Tetrahydrobiopterin L- Tyrosine DOPA Dopamine Adrenaline CH3 Noradrenaline COMT Urine COMT unchanged 5 -6 % MAO MAO Metanephrine 3,4 dihydroxy mandelic acid Metanoepinephrine MAO MAO 4-OH-3 Methoxy Mandelic aldehyde 4-OH-3 Methoxy Mandelic Aldehyde Aldehyde dehydrogenase→ Aldehyde dehydrogenase Vanillyl mandelic acid (VMA)→ nerve terminus → Urine 41% Aldehyde reductase Aldehyde reductase Urine 40% Vanillic acid → Urine 7 % Urine Free or conjugated or acetylated Free or conjugated or acetylated
- 54. FAD linked Monoamine oxidase (MAO) in degradation of Adrenaline and Noradrenaline in mitochondria of liver and target organs Noradrenaline Adrenaline COMT MAO COMT metanephrine Normetanephrine 3,4 dihydroxy mandelic acid MAO COMT Vanillyl mandelic acid (VMA) excreted in urine in the conjugated form ( 3-methoxy-4-hydroxy mandelic acid) MAO= FAD linked Monoamine oxidase (MAO) in mitochondria of liver, stomach ,intestine ,kidney COMT= Catechol-O-methyl transferase (cytosolic) ➢These reactions can occur in almost any order and in any combination in liver or the target tissue .
- 55. Function of FAD linked Monoamine oxidase (MAO) in degradation of Adrenaline and Noradrenaline to VMA :1 • Adrenaline /epinephrine and noradrenaline/ norepinephrine are catabolized (methylation)by cytosolic catechol-O-methyl transferase (COMT) to metanephrine/ normetanephrine which then undergo oxidative deamination by mitochondrial FAD linked Monoamine oxidase (MAO) within the nerve terminus. The major end product is Vanillyl mandelic acid (VMA) = 3-methoxy -4-hydroxy mandelic acid which is excreted in urine in the conjugated form. • Urinary VMA level ( normal 2-6 mg / 24 h ) is increased in Pheochromocytoma – Adrenaline/epinephrine excess and Neuroblastoma –noradrenaline/ norepinephrine excess and useful in the diagnosis tumors of adrenal medulla which cause severe hypertension. • MAO inhibitors : anti-hypertensives, anti-depressants
- 56. Function of FAD linked monoamine oxidase (MAO) in degradation of Adrenaline and noradrenaline to VMA :2 Adrenaline /epinephrine and noradrenaline/ norepinephrine are catabolized by catechol-O-methyl transferase ( COMT ) to metanephrine /normetanephrine which then undergo oxidative deamination by FAD linked Monoamine oxidase (MAO) within the nerve terminus. The major end product is Vanillyl mandelic acid (VMA) = 3- methoxy -4 -hydroxy mandelic acid which is later excreted in urine in the conjugated form.
- 57. Metabolism of Adrenaline and Noradrenaline Liver : Phenylalanine → Tyrosine catalyzed by Phenylalanine hydroxylase ,O2 ,NADPH, Tetrahydrobiopterin Tyrosine enters mitochondria Tyrosine hydroxylase O2 ,NADPH ,Tetrahydrobiopterin Mitochondria DOPA (Dihydroxy Phenylalanine) enters cytoplasm DOPA decarboxylase PLP Cytosol CO2 Dopamine enters chromaffin granules Granulated vesicles of brain cells Dopamine hydroxylase Cu2+, Vit C vesicles or granules of pheochromocytes or nerve endings Noradrenaline enters cytoplasm N –Methyl transferase SAM Cytosol in nerve cells synthesis ends, noradrenaline stored in SAH granulated vesicles Adrenaline Catechol –O–methyl transferase (COMT) SAM Cytosol of liver or target organs of after their synthesis in pheochromocytes→ transported to SAH chromaffin granules for storage Metanephrine FAD dependent monoamine oxidase (MAO) mitochondria of liver or target organs of Vanillyl mandelic acid (VMA)→ nerve terminus→ urine Dopamine, Noradrenaline , Adrenaline, Metaepinephrine and Vanillyl mandelic acid are synthesized in adrenal medulla and sympathetic ganglia.
- 58. Catecholamines Metabolism of Catecholamines Precursors for synthesis Phenylalanine → Tyrosine (Liver : Phenylalanine hydroxylase) O2,NADPH,Tetrahydrobiopterin Site of synthesis Tyrosine is transported to Catecholamines secreting neurons for their synthesis Tissue specificity for synthesis Adrenal medulla ( Adrenaline , Noradrenaline) substantia nigra, coeruleus of brain(Dopamine) Enzymes involved in synthesis DOPA decarboxylase, Dopamine hydroxylase, N-Methyl transferase Storage In the synaptic vesicles Release Ca 2+ dependent release from Synaptic vesicles in response to action potential Receptors and action Adrenaline/epinephrine→ 1, 2, 1, 2 (increase production of c-Amp→ Glucagon) Noradrenaline/norepinephrine → more specific for receptors Dopamine- D1 like ( D1 and D5)→ increase production of c- Amp and D2 like ( D2,D3, D4) dopaminergic receptors → inhibit production of c- Amp Binding to receptors→ dissociation from receptors → Biological response Reuptake Reuptake follows their secretion by the andregenic neurons , which is facilitated by high affinity uptake mechanism for their reutilization or degradation to 3, 4 dihydroxy mandelic acid-inactive metabolite Degradation By enzymes COMT and FAD linked Monoamine oxidase (MAO) → VMA → release in the nerve terminus→ excretion in urine
- 59. Excretory products formed from Metabolic degradation of Catecholamines Excretory products in urine (formed from metabolic degradation of catecholamines) Percentage Unchanged catecholamines 5-6 % Metanephrine (3-methoxy epinephrine) 40 % VMA(3-Methoxy-4-hydroxy Mandelic acid) 41% Vanillic acid (3-Methoxy-4-hydroxy phenyl glycol) 7% Miscellaneous : Metanoepinephrine (3-methoxy Norepinephrine),Catechol - dihydroxy benzene, acetylated derivatives 6% The Urinary metabolites of Catecholamines are excreted mostly as conjugated with sulphates or glucuronides ; sulphate being preferred conjugate form in the human beings.
- 60. Reference values of Adrenaline, noradrenaline and VMA in adults Metabolite Normal plasma Normal urinary excretion In Pheochromocytomas Adrenaline (standing 30 min) < 90 pg/ ml <491 pmol /L (0.5 - 20 g /day) 3 -109 nmols /day increased plasma levels and urinary excretion Noradrenaline (standing 30 min) 125- 700 pg /ml ( 739-4137pmols/L) 15-80 g /day (89 -473 nmols /day) increased plasma levels and urinary excretion Metanephrine(total) 328-1837pg/ml (1.7-9.3 nmol/L) 74-297g /day (375-1506nmols /day) 3 - 112 mg/day Metanephrine(total) - 46 -307 g/g creatinine 26-176 mmol/mol creatinine Normetanephrine/ Metanoepinephrine (Total) :624—3041pg/ml (3.4-16.6nmols /L) 105- 354 g /day (573-1933 nmols /day) Normetanephrine/ Metanoepinephrine - 96 -411 g/g creatinine 59-254 mmol/mol creatinine Urinary excretion of VMA-Vanillyl mandelic acid - 1.4 - 6.5 mg/day (7-33 mols /day) 3.0-8.8 mg/g creatinine 1.7-5.0 mmol/mol creatinine Increased up to 530 mg/day
- 61. Pheochromocytoma • Pheochromocytoma : tumors of adrenal medulla (the chromaffin cells) that secrete high amounts of catecholamines which cause severe hypertension. • Pheochromocytoma tumors may be derived from neural crest cells of sympathetic nervous system (0.1 to 2% cases). • Pheochromocytoma are located in the adrenal gland itself in 85 % of cases. Excluding neuroblastomas, 10% Catecholamine secreting neuroendocrine tumors arise from extra adrenal sympathochromaffin tissue and are called paragangliomas. (located outside the adrenal gland i.e. usually in the abdomen, urinary bladder, neck, thorax, or pelvis )
- 62. Clinical features of Pheochromocytoma :tumors of adrenal medulla (the chromaffin cells) that secrete high amounts of catecholamines which cause severe hypertension 1. Hypertension 2. Impaired glucose tolerance→ hyperglycemia and sometimes glycosuria 3. Palpitation 4. Anxiety 5. Sweating 6. Headache 7. Tremors 8. Nausea 9. Flushing 10. Impending doom 11. Chest or abdominal pain 12. Weight loss 13. Weakness
- 63. Neuroblastoma • Neuroblastoma : is a malignant hemorrhagic tumors of the nerve cells found in adrenal medulla or may be extra- adrenal (60%) secreting high amounts of catecholamines(and their metabolites) . Some Neuroblastoma cells secrete Dopamine and noradrenaline(not Adrenaline). • Location : intraabdominal arising from adrenal gland or the upper abdomen , less frequent locations → chest ,neck , or pelvic regions • Occurrence : chiefly in infants and children • Diagnosis : elevated serum levels of Dopamine , Noradrenaline and hence urinary Homovanillic acid (HVA), Vanillyl mandelic acid (VMA) respectively Anatomical location of the primary tumor in neuroblastomas parallels to the sympathetic nervous system, as predicated from its neurological origin.
- 64. Pheochromocytoma and Neuroblastoma Neuroblastoma: malignant hemorrhagic tumor composed of cells resembling neuroblasts that give rise to cells of the sympathetic system especially adrenal medulla.
- 65. Functions of Dopamine ❖Normal plasma levels of Dopamine in adults (standing 30 min): < 87 pg/ml (<475 pmol/L) ❖Normal urinary excretion of Dopamine in adults : 65-400 g /day (424 -2612 nmols/day) ❖Functions of Dopamine : 1. a major neurotransmitter in nerves that interconnect the nuclei of basal ganglia in the brain ( especially in pyramidal tract , substantia nigra and striatal tract) 2. Control involuntary movements 3. an inhibitor of Prolactin secretion 4. Vasodilator (in periphery) → therefore used in treatment renal failure to stimulate renal blood flow 5. Involved in emotional responses and memory ( as it is found in limbic systems of brain) 6. DOPA and DOPA analogs are useful in management of Parkinson's disease .
- 66. Dopamine and Parkinson's disease ❖Parkinson's disease: 1. Biochemical basis : decreased synthesis of Dopamine due to degeneration of certain parts of brain (substantia nigra, locus coeruleus) in elderly patients 2. Symptoms : a. muscular rigidity b. tremors c. expressionless face d. lethargy e. involuntary movements 3. Management of Parkinson's disease : Administration of DOPA(levodopa)and its analogs ( methyl-dopa, Carbidopa). In brain ,DOPA is decarboxylated to dopamine. Dopamine cannot enter the brain due to the blood brain barrier.
- 67. Degradation of Dopamine Dopamine COMT 3-Methoxy dopamine Di hydroxyphenyl acetic acid COMT Homovanillic acid (HVA) COMT= Catechol-o-methyl transferase (cytosolic) ➢These reactions can occur in almost any order and in any combination in liver or the target tissue . ➢ MAO= FAD linked Monoamine oxidase (MAO) inactivates Serotonin.
- 68. Site of synthesis and functions of Serotonin ❖Site of synthesis of Serotonin : argentaffin cells of gastrointestinal tract, neurons and pineal gland. The brain itself synthesizes 5HT from serotonin. ❖Functions of Serotonin: 1. a neurotransmitter in brain 2. involved in vasoconstriction 3. smooth muscle contraction in bronchioles and arterioles 4. gastrointestinal motility (peristalsis) 5. platelet aggregation 6. Regulation of cerebral activity →excitation→ mood elevator 7. hormonal balance 8. social /sexual behavior 9. sleep /awake cycles 10. appetite and temperature regulation
- 69. Function of FAD linked Monoamine oxidase (MAO) in catabolism of Serotonin • Serotonin undergoes oxidative deamination by FAD linked Monoamine oxidase (MAO) to 5-hydroxy indoleacetic acid (HIAA) which is then excreted in urine in the free form (small amount as conjugated form →esters of sulphate). • FAD linked Monoamine oxidase (MAO): inactivates Serotonin • Monoamine oxidase (MAO) inhibitors (e.g. Iproniazid ) cause mood elevation= anti-depressants, anti-hypertensives • Secretion of 5-hydroxy indoleacetic acid ( HIAA) by Carcinoid tumors is intermittent therefore repeated samples collections and serial measurement are needed for its confirmed diagnosis.
- 70. Function of FAD linked Monoamine oxidase (MAO) in catabolism of Serotonin Tryptophan NADP Tetrahydrobiopterin O2 Tryptophan hydroxylase NADPH +H+ Dihydrobiopterin H2O 5-hydroxy Tryptophan (5HT) PLP Decarboxylase →CO2 5-hydroxy tryptamine(Serotonin) O2 Acetyl CoA FAD-Monoamine oxidase MAO Acetylase NH3 CoA 5-hydroxy indole acetic acid(HIAA) Acetyl Serotonin SAM methyl transferase SAH Melatonin Serotonin is synthesized by neurons ,pineal gland and argentaffin tissue of abdominal cavity using Tryptophan (1-3%). Serotonin undergoes oxidative deamination by FAD linked Monoamine oxidase (MAO) to 5- hydroxy indoleacetic acid ( HIAA).
- 71. Function of FAD linked Monoamine oxidase (MAO) in catabolism of Serotonin Serotonin undergoes oxidative deamination by FAD dependent monoamine oxidase (MAO) to 5- hydroxy indoleacetic acid ( HIAA). Serotonin is synthesized by neurons ,pineal gland and argentaffin tissue of abdominal cavity using Tryptophan (1-3%).
- 72. Modulators(drugs)of FAD linked Monoamine oxidase (MAO) Drug/ Modulator Action on MAO Serotonin levels after therapy Cerebral activity after therapy Therapeutic use N,N –Dimethyl propargylamide Suicidal inhibition Elevated (serotonin→5HT) Excitation→ Psychic stimulant Antidepressant , Parkinson’s disease (-) Deprenyl Suicidal inhibition Elevated (serotonin→5HT) Excitation→ Psychic stimulant Antidepressant , Parkinson’s disease Iproniazid (isopropyl isonicotinylhydrazine) Inhibition Elevated (serotonin→5HT) Excitation→ Psychic stimulant Antidepressant(for depressed patients) Reserpine Activator Decreased (serotonin→5HT ) Decreased→ Psychic Depressant Depressant drug(for aggressive patients) LSD( Lysergic acid diethylamide)competes with serotonin Competitive inhibition Decreased (serotonin→5HT ) Decreased→ Psychic Depressant Depressant drug Amitriptyline ,Trazodone inhibit uptake of serotonin by presynaptic neurons thereby increasing the concentration of Serotonin at serotonergic synapses.
- 73. Carcinoid syndrome ❖Argentaffin cells may grow in small intestine or in appendix into malignant tumors→ argentaffinomas / carcinoid tumors . Carcinoid tumors may also develop from enterochromaffin cells widely distributed in gastrointestinal tract ,biliary duct , gall bladder , pancreatic duct and bronchial tree. ❖Carcinoid syndrome : the clinical features associated with argentaffinomas. ❖Increased serotonin secretion observed in : a. carcinoid tumors in gastrointestinal tract (74%) or respiratory tract(25%) ( bronchus -Oat cell carcinoma of lung) b. Tropical sprue c. Whipple’s disease Diagnosis: elevated serotonin levels in serum, plasma ,whole blood , platelet, urine, serum chromogranin A , and urinary 5-HIAA ✓Platelet Serotonin not affected by patient’s diet (banana ,tomato etc.)
- 74. Clinical features of Carcinoid syndrome ❖Carcinoid syndrome : the clinical features associated with argentaffinomas. ❖In Carcinoid syndrome ,60 % of Tryptophan is diverted to serotonin synthesis against 1 % in normal /physiological conditions resulting in niacin deficiency ( pellagra ). ❖Symptoms of argentaffinomas are due to effects of Serotonin on the smooth muscles. ❖Clinical features/ Symptoms of Carcinoid syndrome include : 1. Flushing (on the face and neck , mediators: histamine , bradykinins , tachykinin ) 2. Sweating 3. Bronchial constriction(respiratory distress and bronchial spasm) → occasionally cyanotic appearance. 4. Intermittent diarrhea 5. Fluctuating hypertension 6. Cardiac lesions ( some may have right-sided heart failure ). Serotonin passing through lungs is destroyed by FAD dependent MAO hence left side is not affected.
- 75. Common sites of Carcinoid tumors Gastrointestinal carcinoid Tumor(yellow-tan) Carcinoid Tumor of Lung Classification of Carcinoid tumors :on their presumed origin from the embryonic Foregut :bronchus, lung , stomach ,duodenum ,pancreas Midgut : ileum, jejunum , appendix , proximal colon Hindgut :distal colon , rectum
- 76. Reference levels of Serotonin and Urinary 5-hydroxy indoleacetic acid (HIAA) in Carcinoid syndrome • Normal plasma Serotonin levels( physiological conditions) : 5-20 mg/ dL (0.2 -2 mols /L ) • Plasma Serotonin levels in carcinoid syndrome ( excessive production of Serotonin) : >40 mg/ dL • Serotonin levels are low with depressive psychosis, Hartnup disease , phenylketonuria, intestinal resection • Normal levels of urinary 5-hydroxy indoleacetic acid(HIAA): < 6mg /day • Urinary 5-hydroxy indoleacetic acid (HIAA) in carcinoid syndrome : > 25 mg /day In metastatic tumor (functioning) and > 350 mg /day in Carcinoid syndrome ➢5-hydroxy indoleacetic acid (HIAA) : a useful maker in Carcinoid syndrome ➢False positive elevation of HIAA: ingestion of serotonin rich food → banana , kiwi, plum, chocolate ,walnuts , avocados and cough medicine guaifenesin) ➢Alcohol , aspirin and certain drugs : suppress 5-HIAA levels
- 77. Biosynthesis of Melatonin Serotonin Acetyl CoA Serotonin N-acetylase Acetylation CoASH N- Acetyl Serotonin S- Adenosyl methionine Methylation CH3 N- Acetyl Serotonin-O-methyltransferase S- Adenosyl homocysteine Melatonin Rate limiting enzyme ❖Synthesis and Secretion of Melatonin by pineal gland is regulated by light. Pineal gland
- 78. Serotonin is methylated to Melatonin in pineal gland In pineal gland, Serotonin is acetylated and then methylated to Melatonin with help of S-adenosyl methionine (SAM) . Melatonin is a neurotransmitter and connected with sleep/ awake cycles ,biological /circadian rhythms and reproductive functions .It is an inhibitor of ACTH and MSH .It has effects on the hypothalamic- pituitary system.
- 79. Functions of Melatonin ❖Melatonin is a hormone of pineal body and peripheral nerves. ❖Functions of Melatonin : 1. Involved in circadian rhythm or diurnal variation( 24hr cyclic process) and plays role in sleep awake cycles. 2. Inhibits production of melanocytes stimulating hormone( MSH) and adrenocorticotropic hormones (ACTH). 3. Some inhibitory of effect on the ovarian functions( mediate in the effect of light on seasonal reproductive cycles). 4. Functions as a neurotransmitter.
- 80. Deficiencies of flavoproteins MAO A and B ❖Deficiency of flavoproteins MAO A: a. Rare occurrence b. Associated with clinical and neurochemical phenotype c. Behavioral disorder-aggressiveness d. Increased plasma and urinary deaminated metabolites of catecholamines e. Increased levels of metanephrine and normetanephrine ❖Deficiency of flavoproteins MAO B: 1. Mild phenotype 2. Increased urinary excretion of phenylethylamine
- 81. FAD linked Metabolic pathway of Glycine Glycine Transaminase Malate ( TCA ) H2O H2O2 Glycine Glyoxylate Oxalate (excreted in urine) NH3-CH2-COO - CHO -COO - Formate → N-Formyl THF Normal urinary oxalic acid level : 20-50mg/day FAD linked Glycine oxidase
- 82. Function of Glycine transaminase and FAD linked Glycine oxidase in the formation of Glyoxylate Glycine oxidase Glycine is reversibly converted to Serine by THF dependent Serine hydroxy methyl transferase . Serine is degraded to Glyoxylate which undergoes transamination by Glycine transaminase to give back Glycine . Glyoxylate is also converted to Oxalate ( excretory products ) and Formate (which enters one carbon pool). FAD linked Glycine transaminase Serine Ethanolamine Glycolate Serine
- 83. Glycine metabolism in Primary hyperoxaluria NAD + Glycine Threonine Glycine synthase THF Glycine oxidase N5N10MTHF NADH + H + N5N10MTHF Serine Pyruvate Glucose CO2+ NH+ 4 Transamination H2O CO2 Serine dehydratase Glycine Transaminase H2O2 Ethanolamine NH3 Glyoxylate Glycolate ½ O2 Oxalate Formate (excreted) THF block in Primary hyperoxaluria N-Formyl THF FAD PLP Threonine aldolase THF Serine hydroxy methyl transferase
- 84. Function of Glycine transaminase and Primary hyperoxaluria • Primary hyperoxaluria is associated with defect in Glycine transaminase to catabolize Glyoxylate( formed by deamination of Glycine) coupled with impairment in glyoxylate oxidation to Formate. • Primary hyperoxaluria is due to defect in protein targeting (i.e. defect in transport of one compound from one compartment to another) . As a result , the enzyme Glycine transaminase ( = Alanine Glyoxylate amino transferase ) is found in mitochondria instead of its normal distribution in hepatic peroxisomes. So enzyme is inactive. (Primary hyperoxaluria Type1= PH1)
- 85. Biochemical characteristics of Primary hyperoxaluria ❖Primary hyperoxaluria is an inborn error in Glycine metabolism , present in early childhood with high urinary excretion of Oxalate and early stone formation. The metabolic defect involves failure to catabolize Glyoxylate ,which therefore gets oxidized to Oxalate and results in urolithiasis, Nephrocalcinosis, and early mortality due to renal failure or hypertension . ❖It is autosomal recessive trait. • Increased excretion of Oxalates up to 600 mg/day, compared to normal up to 50 mg/day .The oxaluria is of endogenous origin due to increased production of Oxalates from Glycine. Oxaluria is unrelated to dietary intake of Oxalates.
- 86. Glycine transaminase in peroxisomes & cytoplasmic Glyoxylate oxidase Primary hyperoxaluria type 1 (PH1 ) is due to protein targeting defect . Normally enzyme Glycine transaminase /Alanine glyoxylate amino transferase is located in hepatic peroxisomes , but in patients with primary hyperoxaluria type 1, it is located in mitochondria/ cytoplasm ,so enzyme is inactive. This leads to increased pool size of Glyoxylate and increased synthesis of endogenous Oxalate . Primary hyperoxaluria Type 2(PH2) is milder condition causing only urolithiasis ,results from deficient activity of cytoplasmic glyoxylate oxidase/reductase . PH1. PH2
- 87. Primary hyperoxaluria type 1 and 2 (PH1 and PH2) Pyruvate Alanine NADPH NADP+ Glycine Glyoxylate Glycolate Oxalic acid 1 2 COOH COOH COOH COOH CH 2 – NH 2 CHO CH 2 OH COOH 1. Glycine transaminase /Alanine glyoxylate amino transferase is located in hepatic peroxisomes , but in patients with Primary hyperoxaluria type 1, it is located in cytoplasm ,so enzyme is inactive. 2. Cytosolic glyoxylate oxidase/reductase - is deficient in activity in Primary hyperoxaluria type 2 .
- 88. Function of Glycine transaminase in Primary hyperoxaluria Type 1 (PH1) and Cytoplasmic Glyoxylate oxidase/reductase in Primary hyperoxaluria 2 (PH2) • Primary hyperoxaluria Type 2(PH2) • results from deficient activity of cytoplasmic Glyoxylate oxidase/reductase. • is milder condition causing urolithiasis ,hematuria and urinary tract infections. • Rarely Nephrocalcinosis and renal failure
- 89. Clinical features of Primary hyperoxaluria ❖Clinical features of Primary hyperoxaluria include : 1. Oxaluria : (increased excretion of urinary oxalate especially Calcium oxalate → stone formation ) 2. Oxalosis (deposition of oxalate in various tissue especially kidney) 3. Urolithiasis(urolith= stones in urinary tract) 4. Renal colic 5. Hematuria 6. Recurrent urinary tract infections 7. Renal failure due to Nephrocalcinosis (presence of calcium deposits in kidney) and hypertension . 8. Extrarenal oxalosis may be seen in heart, blood vessels ,bones etc. 9. Death occurs in childhood or in adults before age< 20 years in the untreated patients from renal failure or hypertension .
- 90. Oxaluria and Urolithiasis in primary hyperoxaluria Oxaluria (increased excretion of urinary) Calcium oxalate →Calcium oxalate stones) Urinary Calcium oxalate crystals in microscopy Urolithiasis(Urolith= Stones in urinary tract) Urinary Calcium oxalate stones The oxaluria is of endogenous origin due to increased production of oxalates from Glycine. Normal urine : 1- 2 Calcium oxalate crystals per high power field Dumb-bell shape Envelope shape
- 91. Oxalosis (deposition of Calcium oxalate in various tissue) in Primary hyperoxaluria Oxalosis involving skin lesions Retinal oxalosis Nephrocalcinosis (presence of calcium deposits in kidney )
- 92. Severe systemic oxalosis In Primary hyperoxaluria ,the metabolic defect involves failure to catabolize Glyoxylate ,which therefore gets oxidized to Oxalate and results in oxalosis. Extrarenal oxalosis may be seen in bones , heart, blood vessels , retina etc. Retinal oxalosis
- 93. Management of Primary hyperoxaluria ❑Management of Primary hyperoxaluria : a) The principle of management of Primary hyperoxaluria is to increase oxalate excretion by increased water intake . b) Try to minimize dietary intake of oxalates by restricting intake of leafy vegetables, sesame seeds ,tea, cocoa, beet root, spinach, rhubarbs etc. c) Dialysis d) combined liver and kidney transplantation ❑In normal individuals ,oxalate can arise from: 1. Glyoxylate metabolism 2. From ingestion of leafy vegetables 3. From ascorbic acid degradation ( very minimal in human beings)
- 94. Function of FAD linked Xanthine oxidase in Uric acid formation ❑FAD linked Xanthine oxidase ( metalloflavoprotein) : • contains Molybdenum and iron . • found in liver and small intestine . • converts hypoxanthine to Xanthine and Xanthine to uric acid. • liberates toxic H2O2 .(Catalase cleaves harmful H2O2 to H2O and O2.) ❑Uric acid is an excretory end product of purine metabolism and can serve as an antioxidant by getting converted nonenzymatically to allantoin .
- 95. Function of FAD linked Xanthine oxidase in Degradation of purine nucleotides in liver Adenosine monophosphate (AMP) H2O AMP deaminase NH4 + Inosine monophosphate (IMP) Adenosine H2O H2O Phospho-monoesterase Adenosinedeaminase Pi NH4 + Inosine Guanosine Guanosine Pi Pi PNP Guanase PNP R-1-P → NH4 + R-1-P Hypoxanthine Xanthosine Guanine H2O+ O2 Pi Xanthine oxidase PNP Guanase H2O2 R-1-P → NH 4 + Xanthine H2O+ O2 Xanthine oxidase H2O2 Uric acid Allopurinol : competitive inhibitor (suicide inhibition) of Flavoprotein Xanthine oxidase is used in management of Gout and Lesch-Nyhan syndrome. Xanthinuria: inborn error of purine metabolism characterized by a complete deficiency of hepatic FAD linked Xanthine oxidase . R-1-P : ribose 1 phosphate PNP: purine nucleoside phosphorylase
- 96. Function of FAD linked Xanthine oxidase in Degradation of purine nucleotides in liver Adenosine monophosphate (AMP) H2O AMP-deaminase NH4 + Inosine monophosphate (IMP) Adenosine H2O H2O Phospho-monoesterase Adenosinedeaminase Pi NH4 + Inosine Guanosine Guanosine Pi Pi PNP Guanase PNP R-1-P → NH4 + R-1-P Hypoxanthine Xanthosine Guanine H2O+ O2 Pi Xanthine oxidase PNP Guanase H2O2 R-1-P → NH 4 + Xanthine H2O+ O2 Xanthine oxidase H2O2 Uric acid Allopurinol : competitive inhibitor (suicide inhibition) of Flavoprotein Xanthine oxidase is used in management of Gout and Lesch-Nyhan syndrome. Xanthinuria: inborn error of purine metabolism characterized by a complete deficiency of hepatic FAD linked Xanthine oxidase . R-1-P : ribose 1 phosphate PNP: purine nucleoside phosphorylase
- 97. FAD-Xanthine oxidase FAD-Xanthine oxidase Function FAD linked Xanthine oxidase in purine metabolism Uric acid is an excretory product of purine metabolism and an antioxidant
- 98. Clinical aspects of Flavoprotein Xanthine oxidase in management of Gout and Lesch-Nyhan syndrome ➢Normal serum uric acid levels = 3-7 mg/dl ➢Daily urinary uric acid excretion = 500-700 mg ➢Gout and Lesch-Nyhan syndrome → hyperuricemia and uricosuria ➢Gout: accumulation of urates in the synovial joints resulting in inflammation and acute arthritis • Allopurinol : competitive inhibitor (suicide inhibition) of Flavoprotein Xanthine oxidase is used in management of Gout and Lesch-Nyhan syndrome to reduce the formation of uric acid in these patients. FAD-Xanthine oxidase Allopurinol Alloxanthin (more potent inhibitor of Xanthine oxidase) Hypoxanthine and Xanthine being more water soluble excreted in urine more easily .
- 99. Gout (a disease associated with overproduction of uric acid) and Allopurinol Allopurinol : competitive inhibitor (suicide inhibition) of Flavoprotein Xanthine oxidase is used in management of Gout to reduce the formation of uric acid in these patients.
- 100. Lesch-Nyhan syndrome and Allopurinol Self mutilation Lesch-Nyhan syndrome is associated with HGPRT deficiency resulting in increased synthesis of purine nucleotides and hence uric acids.( a recessive X-linked trait ) Clinical features : self-mutilation, mental retardation , spastic paraplegia ,aggressive behavior, athetosis Neurological symptoms are due to dependence of brain on salvage pathway of purine metabolism. Biochemical features: Hyperuricemia with gout and urinary lithiasis .Allopurinol (inhibitor of flavoprotein Xanthine oxidase )controls Hyperuricemia but not neurological symptoms.
- 101. Self-mutilation in Lesch-Nyhan syndrome
- 102. Xanthinuria: a complete deficiency of hepatic Flavoprotein Xanthine oxidase ❖Biochemical manifestations of Xanthinuria: 1. Inborn error of purine metabolism characterized by a complete deficiency of hepatic Flavoprotein Xanthine oxidase. 2. Autosomal recessive 3. Rare occurrence 4. Increased plasma concentration of Hypoxanthine and Xanthine and their urinary excretion 5. Xanthine lithiasis 6. Plasma and urine uric acid levels are low
- 103. Xanthinuria: inborn error of purine metabolism characterized by a complete deficiency of hepatic Flavoprotein Xanthine oxidase. Xanthine crystals in urine
- 104. Function of FAD linked Glutathione reductase in detoxification as an antioxidant Glutathione peroxidase detoxifies H2O2 to water while reduced Glutathione (GSH ) is converted to oxidized Glutathione (GS-GS ).The reduced Glutathione can be regenerated by Glutathione reductase utilizing NADPH. Glutathione reductase needs FAD as a coenzyme . This reaction is necessary for removal of lipid peroxides . Riboflavin deficiency is associated with increased lipid peroxidation and oxidative stress . (FAD functions as an antioxidant)
- 105. Flavoproteins function in steroid biosynthesis in conjugation with the cytochrome P450 in mitochondria of adrenal glands :1 ❖The cytochrome P450 enzymes are seen in many tissue ,including adrenal glands ,where they are present both in mitochondria and in microsomes. The mitochondrial cytochrome P450 enzymes utilize NADPH dependent flavoprotein , adrenodoxin reductase and a non- heme iron –sulfur protein, adrenodoxin .They are involved in steroid biosynthesis.
- 106. Flavoproteins function in steroid biosynthesis in conjugation with the cytochrome P450 in mitochondria of adrenal glands :2
- 107. Flavoproteins function in Glucocorticoid biosynthesis in conjugation with the cytochrome P450 in mitochondria of adrenal cortex ❖Common pathway for all Corticosteroids biosynthesis : • Precursor: Cholesterol • Site : Adrenal cortex (mitochondria) • Enzyme : Cytochrome -P-450 side chain cleavage enzyme (monooxygenase) • Requirement for the enzyme : FAD containing flavoprotein Fp , a Fe2S2 protein called adrenodoxin , molecular O2 and NADPH • Cholesterol ester (from cytoplasmic lipid droplets )→free Cholesterol(mitochondria) • Initial reaction in Common pathway for Corticosteroids biosynthesis occurs in inner mitochondrial membrane which involve hydroxylation at C20 and C22 of free Cholesterol by 20,22 desmolase and which then cleaves the side chain to form Pregnenolone : Cytochrome-P-450 side chain cleavage enzyme free cholesterol Pregnenolone NADPH , Fp action of three hydroxylase enzymes molecular O2 Isocaproic aldehyde Cortisol
- 108. Flavoproteins function in biotransformation of drugs in conjugation with the cytochrome P450 enzymes in endoplasmic reticulum of liver Cytochrome P450 enzymes are heme containing enzymes ,localized in endoplasmic reticulum(microsomes) of liver. They are mono- oxygenases. Almost all common drugs are metabolized by flavoprotein dependent Cytochrome P450 enzymes by hydroxylation e.g. Phenobarbital , Warfarin etc. R-H + O2 + NADPH + H+ → ROH + H2O + NADP +
- 109. Flavoproteins function in detoxification in conjugation with the cytochrome P450 in endoplasmic reticulum of liver Most of the oxidation reactions of detoxification are catalyzed by monooxygenase or by flavoprotein dependent cytochrome P450 reductase (mixed function oxidase) in endoplasmic reticulum ( microsomes )of liver. They involve addition of a hydroxyl group to aliphatic or aromatic compounds .e.g. Morphine ,Aniline ,Benzopyrene, Aminopyrine which may represented as : RH + O2 + NADPH → ROH + H2O + NADP+
- 110. Function of FAD dependent methylenetetrahydrofolate reductase in methylation of homocysteine Flavins have also been linked with apoptosis and have homocysteine lowering activity .
- 111. Deficiency manifestations of Riboflavin ❖DeficiencymanifestationsofRiboflavinare: • Uncommon(asRiboflavinhaswide spreaddistributionin foodstuffs) • Mostlyseenalongwith deficienciesotherwatersolublevitamins( Beriberi,pellagra, folicacid)andfatsolublevitaminsK .Thisconditionis calledavitaminosis. • Riboflavindeficiency→tissueconcentrationofFADandFMNfall→deceasedin activitiesofenzymesdependentonFADandFMN • SevereriboflavindeficiencycanaffectconversionofvitaminB6to itscoenzymeand decreasedconversionoftryptophan toniacin(pellagra). ❖SymptomsofDeficiencymanifestationsofRiboflavinaremildandnotlife threatening. SpecificprimaryRiboflavinDeficiencyisdifficulttoachievebecauseof two reason: 1. Riboflavinisassociatedwith proteinin thedietandanydietprovidingproteinwill also providea fairamountofRiboflavin. 2. Recyclingof RiboflavinreleasedfromFADandFMNisextremelyefficient.Therefore onlysmallamountsneedtobeingested.
- 112. Conditions associated with Riboflavin Deficiency ❖Conditions associated with Riboflavin Deficiency are : 1. Anorexia 2. Malnutrition(kwashiorkor) 3. Malabsorption 4. Chronic alcoholism (alcohol interfere with the digestion and absorption of riboflavin) 5. Hypothyroidism and adrenal insufficiency (inhibit the conversion of riboflavin to its coenzymes) 6. Anticancer Drugs Doxorubicin and antimalarial quinacrine therapy 7. Drugs such as barbiturates may cause riboflavin deficiency by inducing microsomal oxidation of riboflavin 8. Riboflavin deficiency may occur in newborn infants with hyperbilirubinemia who are treated by phototherapy (UV light causes photodegradation of Riboflavin) .
- 113. Riboflavin Deficiency in newborn infants with hyperbilirubinemia Riboflavin deficiency may occur in newborn infants with hyperbilirubinemia who are treated by phototherapy (UV light causes photodegradation of Riboflavin) .
- 114. Symptoms of Deficiency manifestations of Riboflavin ❖Symptoms of Deficiency manifestations of Riboflavin are • Cheilosis (fissures at the corners of lips /mouth, chelios = lip in Greek) • Angular stomatitis (inflammation at the corners of mouth) • Glossitis (inflammation of tongue –smooth &purplish/magenta colored, glossa = tongue in Greek) • Seborrheic Dermatitis :Rough, scaly and greasy skin, desquamation around ears ,nose nasolabial folds ,scalp • Hyperemia and edema the pharyngeal and oral mucous membranes, sore throat • Circumcorneal vascularization ( the bulbar conjunctival capillaries is the earliest sign of riboflavin deficiency), watering and burning of eyes, photophobia • Lesions of the genitalia • Normochromic, normocytic anemiawith purebloodred aplasiaofthebonemarrow
- 115. Symptoms of Riboflavin Deficiency manifestations Corneal vascularization Cheilosis (fissures at corner of lips /mouth), Angular stomatitis (inflammation at the corners of mouth) Glossitis (inflammation of tongue –smooth & purplish/magenta colored ) Seborrheic Dermatitis: Rough , scaly and greasy skin, desquamation around ears ,nose nasolabial folds
- 116. Symptoms of Riboflavin Deficiency manifestations in elderly Elderly individuals are more vulnerable to riboflavin deficiency due two reasons : 1. Insufficient intake of diet including riboflavin 2. Impaired Gastrointestinal absorption of diet including riboflavin due to disintegration of intestinal epithelium
- 117. Laboratory assessment of Riboflavin status ❖Assessment of Riboflavin status has been made on the basis of : 1. The dietary intake to overt the signs of hypo- ribovitaminosis 2. Excretion of riboflavin in urine 3. Direct measurement of riboflavin or its metabolitesin plasma or erythrocytes 4. The functionalassayusing activation coefficientof stimulationthe enzyme Glutathionereductaseby FAD 5. Determination of FAD linked Glutathione reductase activity in freshly lyzed erythrocytes(by use of fluorometric method)
- 118. Riboflavin assay • Bioassay procedures • Microbial assay: uses lactobacillus Casei , which requires riboflavin . The production of Lactic acid by bacteria is measured. • Chemical method : colorimetric /fluorometric method : utilizes production of Lumiflavin in presence of visible /ultra violet light in the alkaline solution . Lumiflavin is extracted with chloroform . • Direct measurement of riboflavin ,FMN ,and FAD in plasma or erythrocytes : may be made by HPLC usually with fluorescence detection after protein precipitation or by capillary zone electrophoresis with laser induced fluorescence detection (CZE-LIF).
- 119. Fluorometric method for estimation of Plasma and erythrocytes riboflavin levels Lumiflavin is formed when riboflavin is exposed to ultra violet rays and it emits yellow fluorescence .
- 120. Urinary riboflavin ❖Urinaryriboflavinlevels canbemeasuredwith fluorometric and microbiological procedures but for specificity, is the method of choice. • Excretionofriboflavin in urine:mainlyin freeformand50%in asnucleotidesin urine. Itreflectsdietaryintake. • DailyurinaryExcretionofriboflavin: 0.1-0.4mg(10%-20%ofdietaryintake)
- 121. Diagnosis of Deficiency of Riboflavin by estimation of Erythrocytes Glutathione reductase activity • The most commonly used method for Diagnosis of Deficiency manifestations of Riboflavin : by estimation FAD linked Glutathione reductase activity in freshly lyzed erythrocytes (enzyme based assay). • Most methods measure the rate of change of absorbance at 340 nm caused by oxidation of NADPH and have been automated to give rapid throughputs and CVs of < 2% within run, though some have used fluorescent detection with increased sensitivity . • Reference interval of FAD dependent Glutathione reductase activity in freshly lyzed erythrocytes( by use of fluorometric method): 10-50 g/ dL ( 266 -1330 nmol/L)
- 122. Reference intervals of riboflavin Serum or plasma levels of riboflavin: 4 - 24 g/ dL (106 -638 nmol/L) Erythrocytes levels of riboflavin: 15-30 g/100 gm (266 -1330 nmols/ L) Plasma and erythrocytes riboflavin levels remain constant in severe riboflavin deficiency hence determination of blood is not useful . Guidance reference intervals for the activation coefficient of erythrocytes Glutathione reductase activity by FAD : 1.2 ( adequacy ), 1.21 - 1.4 ( marginal deficiency), >1.4 ( deficiency) Leucocytes and platelets levels of riboflavin: 250 g/100 gm Excretion of riboflavin in urine : mainly in free form and 50% in as nucleotides form . It reflects dietary intake .Daily urinary Excretion of riboflavin : 0.1- 0.4 mg (10% -20% of dietary intake) Excretion of riboflavin in feces: in free and nucleotides form tend to remain constant . Daily Excretion of riboflavin in feces : 500- 700 g ,largely from unabsorbed the bacterial synthesis. Traces of 8- flavin and catabolites are found in feces .
- 123. Newer therapeutic uses of riboflavin ❖Newer therapeutic uses of riboflavin are in : 1. Prophylaxis in migraine attacks 2. In treatment of lactic acidosis caused by the use of reverse transcriptase inhibitors in patients with acquired immune deficiency syndrome or by genetic defects in the mitochondrial respiratory chain such as in Leigh disease.
- 124. Recommended intravenous supply of riboflavin ❖Recommended intravenous supply of riboflavin in adults : 3.6 mg/day ❖Riboflavin in TPN mixture may be subjected to photodegradation ,so bags containing riboflavin should either contain fat emulsion or be covered to provide protection from light.
- 125. Function of FMN dependent in Glyoxylate oxidase in peroxisomes of the photosynthetic cell