Physics powerpoint presentation
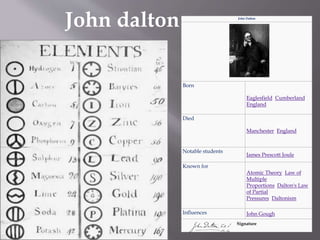
- 1. John Dalton Born 6 September 1766 Eaglesfield, Cumberland, England Died 27 July 1844 (aged 77) Manchester, England Notable students James Prescott Joule Known for Atomic Theory, Law of Multiple Proportions, Dalton's Law of Partial Pressures, Daltonism Influences John Gough Signature John dalton
- 2. Early life John Dalton was born into a Quaker family at Eaglesfield in Cumberland, England. The son of a weaver, he joined his older brother Jonathan at age 15 in running a Quaker school in nearbyKendal. Around 1790 Dalton seems to have considered taking up law or medicine, but his projects were not met with encouragement from his relatives — Dissenters were barred from attending or teaching at English universities — and he remained at Kendal until, in the spring of 1793, he moved to Manchester. Mainly through John Gough, a blind philosopher andpolymath from whose informal instruction he owed much of his scientific knowledge, Dalton was appointed teacher of mathematics and natural philosophy at the "New College" in Manchester, a Dissenting academy. He remained in that position until 1800, when the college's worsening financial situation led him to resign his post and begin a new career in Manchester as a private tutor for mathematics and natural philosophy.
- 3. Five main points of Dalton's atomic theory 1.The atoms of a given element are different from those of any other element; the atoms of different elements can be distinguished from one another by their respective relative atomic weights. 2.All atoms of a given element are identical. 3.Atoms of one element can combine with atoms of other elements to form chemical compounds; a given compound always has the same relative numbers of types of atoms. 4.Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process; a chemical reaction simply changes the way atoms are grouped together. 5.Elements are made of tiny particles called atoms. Dalton proposed an additional "rule of greatest simplicity" that created controversy, since it could not be independently confirmed. When atoms combine in only one ratio, "..it must be presumed to be a binary one, unless some cause appear to the contrary". This was merely an assumption, derived from faith in the simplicity of nature. No evidence was then available to scientists to deduce how many atoms of each element combine to form compound molecules. But this or some other such rule was absolutely necessary to any incipient theory, since one needed an assumed molecular formula in order to calculate relative atomic weights. In any case, Dalton's "rule of greatest simplicity" caused him to assume that the formula for water was OH and ammonia was NH, quite different from our modern understanding. Despite the uncertainty at the heart of Dalton's atomic theory, the principles of the theory survived. To be sure, the conviction that atoms cannot be subdivided, created, or destroyed into smaller particles when they are combined, separated, or rearranged in chemical reactions is inconsistent with the existence of nuclear fusion and nuclear fission, but such processes are nuclear reactions and not chemical reactions. In addition, the idea that all atoms of a given element are identical in their physical and chemical properties is not precisely true, as we now know that different isotopes of an element have slightly varying weights. However, Dalton had created a theory of immense power and importance. Indeed, Dalton's innovation was fully as important for the future of the science as Antoine Laurent Lavoisier's oxygen-based chemistry had been.
- 4. unified atomic mass unit Unit system: SI recognized unit Unit of... mass Symbol: u Unit conversions 1 u in... is equal to... dalton 1 kendrick 0.99888 kg 1.660 538 782(83) × 10 −27 eV/c 2 931.494 028(23) × 10 6
- 5. Atomic mass unit The unified atomic mass unit (symbol: u), also called the dalton (symbol: Da), is a unitused for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound atom of carbon-12 in its nuclear and electronic ground state. The CIPM have categorised it as a "non-SI unit whose values in SI units must be obtained experimentally".[1] It’s value of 1.660538782(83)×10 −27 kg.
- 6. Jöns Jacob Berzelius J. J. Berzelius Born 20 August 1779 Väversunda, Östergötland, Sweden Died 7 August 1848 (aged 68) Stockholm, Sweden Nationality Sweden Fields Chemistry Institutions Karolinska Institute Alma mater Uppsala University Doctoral advisor Johann Afzelius Doctoral students James Finlay Weir Johnston Heinrich Rose Known for Law of constant proportions Chemical notation Silicon Selenium Thorium Cerium Notable awards Copley medal Biography Berzelius was born at Väversunda in Östergötland in Sweden. He lost both his parents at an early age. He was taken care of by relatives in Linköping where he attended the school today known as Katedralskolan. Thereafter he enrolled at the Uppsala University where he learned the profession of medical doctor from 1796 to 1801. He was taught chemistry byAnders Gustaf Ekeberg, the discoverer of tantalum. He worked as apprentice in a pharmacy and with a physician in the Medevi mineral springs. During this time he conducted analysis of the spring water. For his medical studies he examined the influence of galvanic current on several diseases and graduated as M.D. in 1802. He worked as physician near Stockholm until the mine owner Wilhelm Hisinger discovered his analytical abilities and provided him with a laboratory.
- 7. In 1807 Berzelius was appointed professor in chemistry and pharmacy at the Karolinska Institute. In 1808, he was elected a member of the Royal Swedish Academy of Sciences. At this time, the Academy had been stagnating for a number of years, since the era ofromanticism in Sweden had led to less interest in the sciences. In 1818, Berzelius was elected the Academy's secretary, and held the post until 1848. During Berzelius' tenure, he is credited with revitalising the Academy and bringing it into a second golden era, the first being the astronomer Pehr Wilhelm Wargentin's period as secretary (1749-1783). In 1837, he was also elected a member of the Swedish Academy, on chair number 5. Discovery of elements A polycrystalline siliconrod made by the Siemens process Berzelius is credited with identifying the chemical elements silicon, selenium, thorium, and cerium. Students working in Berzelius's laboratory also discovered lithium, and vanadium. New chemical terms Daguerreotype of Berzelius. Berzelius is also credited with originating the chemical terms "catalysis", "polymer", "isomer" and "allotrope", although his original definitions differ dramatically from modern usage. For example, he coined the term "polymer" in 1833 to describe organic compounds which shared identical empirical formulas but differed in overall molecular weight, the larger of the compounds being described as "polymers" of the smallest. According to this (now obsolete) definition, glucose(C6H12O6) would be a polymer of formaldehyde (CH2O).
- 8. Family Statue of Berzelius in the center ofBerzelii Park, Stockholm In 1818 Berzelius was ennobled by King Carl XIV Johan; in 1835, at the age of 56, he married Elisabeth Poppius, the 24- year old daughter of a Swedish cabinet minister, and in the same year was elevated to friherre.[3] Berzeliusskolan, a school situated next to his alma mater, Katedralskolan, is named for him. In 1939 his portrait appeared on a series of postage stamps commemorating the bicentenary of the founding of the Swedish Academy of Sciences. He died on 7 August 1848 at his home in Stockholm, where he had lived since 1806..[4] Chemical compound A chemical compound is a pure chemical substance consisting of two or more different chemical elements[1][2][3] that can be separated into simpler substances by chemical reactions.[4] Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms[3] that are held together in a defined spatial arrangement by chemical bonds. Chemical compounds can be molecularcompounds held together by covalent bonds, salts held together by ionic bonds, intermetallic compounds held together by metallic bonds, orcomplexes held together by coordinate covalent bonds. Pure chemical elements are not considered chemical compounds, even if they consist of molecules which contain only multiple atoms of a single element (such as H2, S8, etc.),[5] which are called diatomic molecules orpolyatomic molecules.
- 9. Elementary concepts Characteristic properties of compounds: 1. Elements in a compound are present in a definite proportion Example- 2 atoms of hydrogen + 1 atom of oxygen becomes 1 molecule of compound-water. 2. Compounds have a definite set of properties Elements of the compound do not retain their original properties. Example- Hydrogen(element{which is combustible and non-supporter of combustion}) + Oxygen(element{which is non-combustible and supporter of combustion}) becomes Water(compound{which is non-combustible and non- supporter of combustion}) 3. Elements in a compound cannot be separated by physical methods. Valency is the number of hydrogen atoms which can combine with one atom of the element forming a compound. Formula Chemists describe compounds using formulas in various formats. For compounds that exist as molecules, the formula for the molecular unit is shown. For polymeric materials, such as minerals and many metal oxides, the empirical formula is normally given, e.g. NaCl for table salt. The elements in a chemical formula are normally listed in a specific order, called the Hill system. In this system, the carbon atoms (if there are any) are usually listed first, any hydrogen atoms are listed next, and all other elements follow in alphabetical order. If the formula contains no carbon, then all of the elements, including hydrogen, are listed alphabetically. There are, however, several important exceptions to the normal rules. For ionic compounds, the positive ion is almost always listed first and the negative ion is listed second. For oxides, oxygen is usually listed last.
- 10. Chemical structure A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as diatomic oxygen or nitrogen molecules, to the very complex, such as protein or DNA molecules. Molecular geometry can be roughly represented using a structural formula. Electronic structure describes the occupation of a molecule's molecular orbitals. Ions For ions, the charge on a particular atom may be denoted with a right-hand superscript. For example Na+, or Cu2+. The total charge on a charged molecule or a polyatomic ion may also be shown in this way. For example: hydronium, H3O+ or sulfate, SO4 2−. For more complex ions, brackets [ ] are often used to enclose the ionic formula, as in [B12H12]2−, which is found in compounds such asCs2[B12H12]. Parentheses ( ) can be nested inside brackets to indicate a repeating unit, as in [Co(NH3)6]3+. Here (NH3)6 indicates that the ion contains six NH3 groups, and [ ] encloses the entire formula of the ion with charge +3. Isotopes Although isotopes are more relevant to nuclear chemistry or stable isotope chemistry than to conventional chemistry, different isotopes may be indicated with a left-hand superscript in a chemical formula. For example, the phosphate ion containing radioactive phosphorus-32 is32PO4 3-. Also a study involving stable isotope ratios might include the molecule 18O16O. A left-hand subscript is sometimes used redundantly to indicate the atomic number. For example, 8O2 for dioxygen, and 16 8O2 for the most abundant isotopic species of dioxygen. This is convenient when writing equations for nuclear reactions, in order to show the balance of charge more clearly.
- 11. Isobutane Molecular formula: C4H10 Semi-structural formula: (CH3)3CH Butane Molecular formula: C4H10 Semi-structural formula: CH3CH2CH2CH3 Chemical symbol A chemical symbol is a 1- or 2-letter internationally agreed code for a chemical element, usually derived from the name of the element, often in Latin. The first letter, only, is capitalised. For example, "He" is the symbol for helium (English name, not known in ancient Roman times), "Pb" for lead (plumbum in Latin), "W" for tungsten (wolfram in German, not known in Roman times). Temporary symbols assigned to newly or not-yet synthesized elements use 3-letter symbols. For example, "Uno" was the temporary symbol for Hassium which had the temporary name of Unniloctium. Chemical symbols may be modified by the use of superscripts or subscripts to specify a particular isotope of an atom. Additionally superscripts may be used to indicate the ionization or oxidation state of an element. Attached subscripts or superscripts specifying a nucleotide or molecule have the following meanings and positions: The nucleon number (mass number) is shown in the left superscript position (e.g., 14N) The proton number (atomic number) may be indicated in the left subscript position (e.g., 64Gd) If necessary, a state of ionization or an excited state may be indicated in the right superscript position (e.g., state of ionization Ca2+). Inastronomy, non-ionised atomic hydrogen is often known as "HI", and ionised hydrogen as "HII".[1] The number of atoms of an element in a molecule or chemical compound is shown in the right subscript position (e.g., N2 or Fe2O3) A radical is indicated by a dot on the right side (e.g., Cl· for a chloride radical)
