IA on effect of different transition metal (homogenous catalyst) on the decomposition of H2O2 measured using a pressure sensor.
•
0 j'aime•246 vues
IA on effect of different transition metal (homogenous catalyst) on the decomposition of H2O2 measured using a pressure sensor.
Signaler
Partager
Signaler
Partager
Télécharger pour lire hors ligne
Recommandé
IA on effectiveness of different types of catalysts MnO2 vs Fe(NO3)3 on the r...

IA on effectiveness of different types of catalysts MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.
IA on effect of temperature of NaOH on the rate of hydrogen production, and f...

IA on effect of temperature of NaOH on the rate of hydrogen production, and finding Ea for reaction between aluminium and sodium hydroxide measured using a pressure sensor.
IA on effect of different transition metal on enzyme catalase (yeast extract)...

IA on effect of different transition metal on enzyme catalase (yeast extract) on the rate of decomposition of H2O2 measured using a pressure sensor.
Recommandé
IA on effectiveness of different types of catalysts MnO2 vs Fe(NO3)3 on the r...

IA on effectiveness of different types of catalysts MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.
IA on effect of temperature of NaOH on the rate of hydrogen production, and f...

IA on effect of temperature of NaOH on the rate of hydrogen production, and finding Ea for reaction between aluminium and sodium hydroxide measured using a pressure sensor.
IA on effect of different transition metal on enzyme catalase (yeast extract)...

IA on effect of different transition metal on enzyme catalase (yeast extract) on the rate of decomposition of H2O2 measured using a pressure sensor.
IA on effect of inhibitor concentration copper on enzyme catalase (yeast extr...

IA on effect of inhibitor concentration copper on enzyme catalase (yeast extract) on the rate of decomposition of H2O2 measured using a pressure sensor.
Temperature fields during the development of combustion in a rapid compressio...

J. Clarkson, J.F. Griffiths, J.P. MacNamara, & B.J. Whitaker, “Temperature Fields During the Development of Combustion in a Rapid Compression Machine”, Combustion and Flame, 125, 1162-1175, 2001.
IB Chemistry on Equilibrium Constant, Kc and Reaction Quotient, Qc.

IB Chemistry on Equilibrium Constant, Kc and Reaction Quotient, Qc.
IB Chemistry Order Reaction, Rate Law and Half life

IB Chemistry Order Reaction, Rate Law and Half life
Conductivity measurement in CSTR water-base(according to temperature)

this report made by koya university student of chemical engineering (shwan sarwan ).
Vapour-Liquid and Solid-Vapour-Liquid Equilibria of the System (CO2 + H2) at ...

Vapour-Liquid and Solid-Vapour-Liquid Equilibria of the System (CO2 + H2) at ...UK Carbon Capture and Storage Research Centre
Presentation given by Dr David Vega-Maza from University of Aberdeen on "Vapour-Liquid and Solid-Vapour-Liquid Equilibria of the System (CO2 + H2) at Temperatures Between (218 and 303) K and at Pressures up to 15 MPa" in the Effects of Impurities Technical Session at the UKCCSRC Biannual Meeting - CCS in the Bigger Picture - held in Cambridge on 2-3 April 2014The phase rule

THE PHASE RULE
phase rule
degree of freedom in mixture
one component system
two component system
pressure temperature diagram sulfur hydrogen
eutectic eutectoid mixture
Algorithm to find the composition of air at temperatures 200-9000 K

This report contains the theory behind the algorithm to find the composition of air at temperatures 200-9000 K. A C++ program for the same is hosted at my profile on GitHub.com/kcavatar.
Chemical engineering thermo dynamics Ii Jntu Model Paper{Www.Studentyogi.Com}

Chemical engineering thermo dynamics Ii Jntu Model Paper{Www.Studentyogi.Com}
Ptme8201 engineering thermodynamics uq - april may 2014

ENGINEERING THERMODYNAMICS ANNA UNIVERSITY QUESTION PAPER MAY 2014
Liquid liquid equilibrium for the ternary system of isopropyl acetate 2 propa...

Liquid liquid equilibrium for the ternary system of isopropyl acetate 2 propa...Josemar Pereira da Silva
Liquid Liquid equilibriumAdvanced Chemical Engineering Thermodynamics-31-July-2016

The slides are good for advanced undergraduate students.
IA on effect of inhibitor concentration lead on enzyme catalase (yeast extrac...

IA on effect of inhibitor concentration lead on enzyme catalase (yeast extract) on the rate of decomposition of H2O2 measured using a pressure sensor.
IA on effect of different inhibitor on enzyme catalase (potato extract) on th...

IA on effect of different inhibitor on enzyme catalase (potato extract) on the rate of decomposition of H2O2 measured using a pressure sensor.
Contenu connexe
Tendances
IA on effect of inhibitor concentration copper on enzyme catalase (yeast extr...

IA on effect of inhibitor concentration copper on enzyme catalase (yeast extract) on the rate of decomposition of H2O2 measured using a pressure sensor.
Temperature fields during the development of combustion in a rapid compressio...

J. Clarkson, J.F. Griffiths, J.P. MacNamara, & B.J. Whitaker, “Temperature Fields During the Development of Combustion in a Rapid Compression Machine”, Combustion and Flame, 125, 1162-1175, 2001.
IB Chemistry on Equilibrium Constant, Kc and Reaction Quotient, Qc.

IB Chemistry on Equilibrium Constant, Kc and Reaction Quotient, Qc.
IB Chemistry Order Reaction, Rate Law and Half life

IB Chemistry Order Reaction, Rate Law and Half life
Conductivity measurement in CSTR water-base(according to temperature)

this report made by koya university student of chemical engineering (shwan sarwan ).
Vapour-Liquid and Solid-Vapour-Liquid Equilibria of the System (CO2 + H2) at ...

Vapour-Liquid and Solid-Vapour-Liquid Equilibria of the System (CO2 + H2) at ...UK Carbon Capture and Storage Research Centre
Presentation given by Dr David Vega-Maza from University of Aberdeen on "Vapour-Liquid and Solid-Vapour-Liquid Equilibria of the System (CO2 + H2) at Temperatures Between (218 and 303) K and at Pressures up to 15 MPa" in the Effects of Impurities Technical Session at the UKCCSRC Biannual Meeting - CCS in the Bigger Picture - held in Cambridge on 2-3 April 2014The phase rule

THE PHASE RULE
phase rule
degree of freedom in mixture
one component system
two component system
pressure temperature diagram sulfur hydrogen
eutectic eutectoid mixture
Algorithm to find the composition of air at temperatures 200-9000 K

This report contains the theory behind the algorithm to find the composition of air at temperatures 200-9000 K. A C++ program for the same is hosted at my profile on GitHub.com/kcavatar.
Chemical engineering thermo dynamics Ii Jntu Model Paper{Www.Studentyogi.Com}

Chemical engineering thermo dynamics Ii Jntu Model Paper{Www.Studentyogi.Com}
Ptme8201 engineering thermodynamics uq - april may 2014

ENGINEERING THERMODYNAMICS ANNA UNIVERSITY QUESTION PAPER MAY 2014
Liquid liquid equilibrium for the ternary system of isopropyl acetate 2 propa...

Liquid liquid equilibrium for the ternary system of isopropyl acetate 2 propa...Josemar Pereira da Silva
Liquid Liquid equilibriumAdvanced Chemical Engineering Thermodynamics-31-July-2016

The slides are good for advanced undergraduate students.
Tendances (20)
IA on effect of inhibitor concentration copper on enzyme catalase (yeast extr...

IA on effect of inhibitor concentration copper on enzyme catalase (yeast extr...
Temperature fields during the development of combustion in a rapid compressio...

Temperature fields during the development of combustion in a rapid compressio...
IB Chemistry on Equilibrium Constant, Kc and Reaction Quotient, Qc.

IB Chemistry on Equilibrium Constant, Kc and Reaction Quotient, Qc.
IB Chemistry Order Reaction, Rate Law and Half life

IB Chemistry Order Reaction, Rate Law and Half life
Conductivity measurement in CSTR water-base(according to temperature)

Conductivity measurement in CSTR water-base(according to temperature)
Vapour-Liquid and Solid-Vapour-Liquid Equilibria of the System (CO2 + H2) at ...

Vapour-Liquid and Solid-Vapour-Liquid Equilibria of the System (CO2 + H2) at ...
Algorithm to find the composition of air at temperatures 200-9000 K

Algorithm to find the composition of air at temperatures 200-9000 K
Chemical engineering thermo dynamics Ii Jntu Model Paper{Www.Studentyogi.Com}

Chemical engineering thermo dynamics Ii Jntu Model Paper{Www.Studentyogi.Com}
Ptme8201 engineering thermodynamics uq - april may 2014

Ptme8201 engineering thermodynamics uq - april may 2014
Liquid liquid equilibrium for the ternary system of isopropyl acetate 2 propa...

Liquid liquid equilibrium for the ternary system of isopropyl acetate 2 propa...
Advanced Chemical Engineering Thermodynamics-31-July-2016

Advanced Chemical Engineering Thermodynamics-31-July-2016
Similaire à IA on effect of different transition metal (homogenous catalyst) on the decomposition of H2O2 measured using a pressure sensor.
IA on effect of inhibitor concentration lead on enzyme catalase (yeast extrac...

IA on effect of inhibitor concentration lead on enzyme catalase (yeast extract) on the rate of decomposition of H2O2 measured using a pressure sensor.
IA on effect of different inhibitor on enzyme catalase (potato extract) on th...

IA on effect of different inhibitor on enzyme catalase (potato extract) on the rate of decomposition of H2O2 measured using a pressure sensor.
REDOX TITRATION.pdf

Redox titration, Reduction & Oxidation, Theory & Principle, Redox indicator, Permanganometry, Iodimetry, Iodometry, Dichrometry, Cerimetry, Bromimetry, Titanometry, Titration involving Potassium iodate
Reduction - Oxidation Titrations Theory and Application.ppt

Reduction - Oxidation Titrations
Theory and Application
Redox Reaction and Electrochemical Cell (Reaksi Redoks dan Sel Elektrokimia)

Redox Reaction and Electrocemicall Cell
chapter8redoxreactionsppt.pdf

This chapter tell you about the reduction in the Oxidation reaction there he is revolutions their transfer of ions and also about the oxidizing agent in the reducing agent
Class 11 Chapter 8 Redox Reactions.pptx

Hxssshshsbdbbbsbbsbssbbbhhshdhhddhhddddbbddbdbbbsbsbbbbsssssbssssssssbsbsbs
Similaire à IA on effect of different transition metal (homogenous catalyst) on the decomposition of H2O2 measured using a pressure sensor. (20)
IA on effect of inhibitor concentration lead on enzyme catalase (yeast extrac...

IA on effect of inhibitor concentration lead on enzyme catalase (yeast extrac...
IA on effect of different inhibitor on enzyme catalase (potato extract) on th...

IA on effect of different inhibitor on enzyme catalase (potato extract) on th...
Reduction - Oxidation Titrations Theory and Application.ppt

Reduction - Oxidation Titrations Theory and Application.ppt
Redox Reaction and Electrochemical Cell (Reaksi Redoks dan Sel Elektrokimia)

Redox Reaction and Electrochemical Cell (Reaksi Redoks dan Sel Elektrokimia)
Plus de Lawrence kok
IA on effect of duration on efficiency of immobilized enzyme amylase (yeast e...

IA on effect of duration on efficiency of immobilized enzyme amylase (yeast extract) in alginate beads, in starch hydrolysis.
IA on efficiency of immobilized enzyme amylase (yeast extract) in alginate be...

IA on efficiency of immobilized enzyme amylase (yeast extract) in alginate beads, in starch hydrolysis.
IA on efficiency of immobilized enzyme amylase (yeast extract) in alginate be...

IA on efficiency of immobilized enzyme amylase (yeast extract) in alginate beads, in starch hydrolysis.
IA on effect of duration on the efficiency of immobilized enzyme amylase (fun...

IA on effect of duration on the efficiency of immobilized enzyme amylase (fungal extract) in alginate beads, in starch hydrolysis.
IA on efficiency of immobilized enzyme amylase (fungal extract) in alginate b...

IA on efficiency of immobilized enzyme amylase (fungal extract) in alginate beads, in starch hydrolysis.
IA on efficiency of immobilized enzyme amylase (fungal extract) in alginate b...

IA on efficiency of immobilized enzyme amylase (fungal extract) in alginate beads, in starch hydrolysis.
IA on effect of duration on efficiency of immobilized MnO2 in alginate beads ...

IA on effect of duration on efficiency of immobilized MnO2 in alginate beads (3%SA with 2%CC).
IA on effect of concentration of sodium alginate and calcium chloride in maki...

IA on effect of concentration of sodium alginate and calcium chloride in making alginate beads.
IA on effect of temperature on polyphenol (tannins) of white wine, using pota...

IA on effect of temperature on polyphenol (tannins) of white wine, using potassium permanganate titration (Lowenthal permanganate).
IA on effect of temperature on polyphenol (tannins) of green tea, using potas...

IA on effect of temperature on polyphenol (tannins) of green tea, using potassium permanganate titration (Lowenthal permanganate).
IA on effect of duration (steeping time) on polyphenol (tannins) of tea, usin...

IA on effect of duration (steeping time) on polyphenol (tannins) of tea, using potassium permanganate titration.
IA on polyphenol (tannins) quantification between green and black tea using p...

IA on polyphenol (tannins) quantification between green and black tea using potassium permanganate titration (Lowenthal permanganate).
IA on temperature on polyphenol (tannins strawberry) quantification using pot...

IA on temperature on polyphenol (tannins strawberry) quantification using potassium permanganate titration (Lowenthal permanganate).
IA on temperature on polyphenol (tannins apple cider) quantification using po...

IA on temperature on polyphenol (tannins apple cider) quantification using potassium permanganate titration (Lowenthal permanganate).
IA on effect of temperature on polyphenol (tannins) quantification using pota...

IA on effect of temperature on polyphenol (tannins) quantification using potassium permanganate titration (Lowenthal permanganate).
IA on polyphenol quantification using potassium permanganate titration (Lowen...

IA on polyphenol quantification using potassium permanganate titration (Lowenthal permanganate).
IA on rate of hydrolysis of aspirin at different temperature, measured using ...

IA on rate of hydrolysis of aspirin at different temperature, measured using visible spectrophotometer.
IA on hydrolysis of aspirin in water, duration over 5 days, measured using vi...

IA on hydrolysis of aspirin in water, duration over 5 days, measured using visible spectrophotometer.
IA on aspirin hydrolysis in different HCI concentration (0.0625 -1M), measure...

IA on aspirin hydrolysis in different HCI concentration (0.0625 -1M), measured using visible spectrophotometer.
IA on aspirin hydrolysis in different medium, water vs acid (1M) medium, meas...

IA on aspirin hydrolysis in different medium, water vs acid (1M) medium, measured using visible spectrophotometer.
Plus de Lawrence kok (20)
IA on effect of duration on efficiency of immobilized enzyme amylase (yeast e...

IA on effect of duration on efficiency of immobilized enzyme amylase (yeast e...
IA on efficiency of immobilized enzyme amylase (yeast extract) in alginate be...

IA on efficiency of immobilized enzyme amylase (yeast extract) in alginate be...
IA on efficiency of immobilized enzyme amylase (yeast extract) in alginate be...

IA on efficiency of immobilized enzyme amylase (yeast extract) in alginate be...
IA on effect of duration on the efficiency of immobilized enzyme amylase (fun...

IA on effect of duration on the efficiency of immobilized enzyme amylase (fun...
IA on efficiency of immobilized enzyme amylase (fungal extract) in alginate b...

IA on efficiency of immobilized enzyme amylase (fungal extract) in alginate b...
IA on efficiency of immobilized enzyme amylase (fungal extract) in alginate b...

IA on efficiency of immobilized enzyme amylase (fungal extract) in alginate b...
IA on effect of duration on efficiency of immobilized MnO2 in alginate beads ...

IA on effect of duration on efficiency of immobilized MnO2 in alginate beads ...
IA on effect of concentration of sodium alginate and calcium chloride in maki...

IA on effect of concentration of sodium alginate and calcium chloride in maki...
IA on effect of temperature on polyphenol (tannins) of white wine, using pota...

IA on effect of temperature on polyphenol (tannins) of white wine, using pota...
IA on effect of temperature on polyphenol (tannins) of green tea, using potas...

IA on effect of temperature on polyphenol (tannins) of green tea, using potas...
IA on effect of duration (steeping time) on polyphenol (tannins) of tea, usin...

IA on effect of duration (steeping time) on polyphenol (tannins) of tea, usin...
IA on polyphenol (tannins) quantification between green and black tea using p...

IA on polyphenol (tannins) quantification between green and black tea using p...
IA on temperature on polyphenol (tannins strawberry) quantification using pot...

IA on temperature on polyphenol (tannins strawberry) quantification using pot...
IA on temperature on polyphenol (tannins apple cider) quantification using po...

IA on temperature on polyphenol (tannins apple cider) quantification using po...
IA on effect of temperature on polyphenol (tannins) quantification using pota...

IA on effect of temperature on polyphenol (tannins) quantification using pota...
IA on polyphenol quantification using potassium permanganate titration (Lowen...

IA on polyphenol quantification using potassium permanganate titration (Lowen...
IA on rate of hydrolysis of aspirin at different temperature, measured using ...

IA on rate of hydrolysis of aspirin at different temperature, measured using ...
IA on hydrolysis of aspirin in water, duration over 5 days, measured using vi...

IA on hydrolysis of aspirin in water, duration over 5 days, measured using vi...
IA on aspirin hydrolysis in different HCI concentration (0.0625 -1M), measure...

IA on aspirin hydrolysis in different HCI concentration (0.0625 -1M), measure...
IA on aspirin hydrolysis in different medium, water vs acid (1M) medium, meas...

IA on aspirin hydrolysis in different medium, water vs acid (1M) medium, meas...
Dernier
The French Revolution Class 9 Study Material pdf free download

The French Revolution, which began in 1789, was a period of radical social and political upheaval in France. It marked the decline of absolute monarchies, the rise of secular and democratic republics, and the eventual rise of Napoleon Bonaparte. This revolutionary period is crucial in understanding the transition from feudalism to modernity in Europe.
For more information, visit-www.vavaclasses.com
Normal Labour/ Stages of Labour/ Mechanism of Labour

Normal labor is also termed spontaneous labor, defined as the natural physiological process through which the fetus, placenta, and membranes are expelled from the uterus through the birth canal at term (37 to 42 weeks
CACJapan - GROUP Presentation 1- Wk 4.pdf

Macroeconomics- Movie Location
This will be used as part of your Personal Professional Portfolio once graded.
Objective:
Prepare a presentation or a paper using research, basic comparative analysis, data organization and application of economic information. You will make an informed assessment of an economic climate outside of the United States to accomplish an entertainment industry objective.
A Survey of Techniques for Maximizing LLM Performance.pptx

A Survey of Techniques for Maximizing LLM Performance
How to Make a Field invisible in Odoo 17

It is possible to hide or invisible some fields in odoo. Commonly using “invisible” attribute in the field definition to invisible the fields. This slide will show how to make a field invisible in odoo 17.
TESDA TM1 REVIEWER FOR NATIONAL ASSESSMENT WRITTEN AND ORAL QUESTIONS WITH A...

TESDA TM1 REVIEWER FOR NATIONAL ASSESSMENT WRITTEN AND ORAL QUESTIONS WITH ANSWERS.
Acetabularia Information For Class 9 .docx

Acetabularia acetabulum is a single-celled green alga that in its vegetative state is morphologically differentiated into a basal rhizoid and an axially elongated stalk, which bears whorls of branching hairs. The single diploid nucleus resides in the rhizoid.
How libraries can support authors with open access requirements for UKRI fund...

How libraries can support authors with open access requirements for UKRI funded books
Wednesday 22 May 2024, 14:00-15:00.
Unit 8 - Information and Communication Technology (Paper I).pdf

This slides describes the basic concepts of ICT, basics of Email, Emerging Technology and Digital Initiatives in Education. This presentations aligns with the UGC Paper I syllabus.
special B.ed 2nd year old paper_20240531.pdf

Instagram:-
https://instagram.com/special_education_needs_01?igshid=YmMyMTA2M2Y=
WhatsApp:-
https://chat.whatsapp.com/JVakNIYlSV94x7bwunO3Dc
YouTube:-
https://youtube.com/@special_education_needs
Teligram :- https://t.me/special_education_needs
Slide Shere :-
https://www.slideshare.net/shabnambano20?utm_campaign=profiletracking&utm_medium=sssite&utm_source=ssslideview
Biological Screening of Herbal Drugs in detailed.

Biological screening of herbal drugs: Introduction and Need for
Phyto-Pharmacological Screening, New Strategies for evaluating
Natural Products, In vitro evaluation techniques for Antioxidants, Antimicrobial and Anticancer drugs. In vivo evaluation techniques
for Anti-inflammatory, Antiulcer, Anticancer, Wound healing, Antidiabetic, Hepatoprotective, Cardio protective, Diuretics and
Antifertility, Toxicity studies as per OECD guidelines
Supporting (UKRI) OA monographs at Salford.pptx

How libraries can support authors with open access requirements for UKRI funded books
Wednesday 22 May 2024, 14:00-15:00.
The Challenger.pdf DNHS Official Publication

Read| The latest issue of The Challenger is here! We are thrilled to announce that our school paper has qualified for the NATIONAL SCHOOLS PRESS CONFERENCE (NSPC) 2024. Thank you for your unwavering support and trust. Dive into the stories that made us stand out!
Digital Artifact 2 - Investigating Pavilion Designs

Digital Artifact 2 - Pavilions
NGV Architecture Commission Competition
MPavilion Commission Competition
Other Pavilion Designs
"Protectable subject matters, Protection in biotechnology, Protection of othe...

Protectable subject matters, Protection in biotechnology, Protection of other biological materials, Ownership and period of protection
Dernier (20)
The French Revolution Class 9 Study Material pdf free download

The French Revolution Class 9 Study Material pdf free download
Normal Labour/ Stages of Labour/ Mechanism of Labour

Normal Labour/ Stages of Labour/ Mechanism of Labour
A Survey of Techniques for Maximizing LLM Performance.pptx

A Survey of Techniques for Maximizing LLM Performance.pptx
Multithreading_in_C++ - std::thread, race condition

Multithreading_in_C++ - std::thread, race condition
TESDA TM1 REVIEWER FOR NATIONAL ASSESSMENT WRITTEN AND ORAL QUESTIONS WITH A...

TESDA TM1 REVIEWER FOR NATIONAL ASSESSMENT WRITTEN AND ORAL QUESTIONS WITH A...
How libraries can support authors with open access requirements for UKRI fund...

How libraries can support authors with open access requirements for UKRI fund...
Unit 8 - Information and Communication Technology (Paper I).pdf

Unit 8 - Information and Communication Technology (Paper I).pdf
Digital Artifact 2 - Investigating Pavilion Designs

Digital Artifact 2 - Investigating Pavilion Designs
"Protectable subject matters, Protection in biotechnology, Protection of othe...

"Protectable subject matters, Protection in biotechnology, Protection of othe...
IA on effect of different transition metal (homogenous catalyst) on the decomposition of H2O2 measured using a pressure sensor.
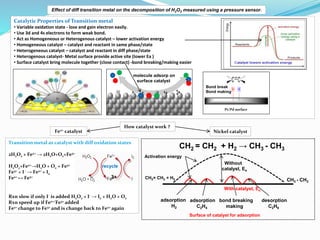
- 1. Pt/Pd surface Catalytic Properties of Transition metal • Variable oxidation state - lose and gain electron easily. • Use 3d and 4s electrons to form weak bond. • Act as Homogeneous or Heterogenous catalyst – lower activation energy • Homogeneous catalyst – catalyst and reactant in same phase/state • Heterogeneous catalyst – catalyst and reactant in diff phase/state • Heterogenous catalyst- Metal surface provide active site (lower Ea ) • Surface catalyst bring molecule together (close contact) -bond breaking/making easier Transition metal as catalyst with diff oxidation states 2H2O2 + Fe2+ → 2H2O+O2+Fe3+ H2O2+Fe2+→H2O + O2 + Fe3+ Fe3+ + I - → Fe2+ + I2 Fe2+ ↔ Fe3+ Rxn slow if only I- is added H2O2 + I- → I2 + H2O + O2 Rxn speed up if Fe2+/Fe3+ added Fe2+ change to Fe3+ and is change back to Fe2+ again recycle molecule adsorp on surface catalyst Pt/Pd surface Bond break Bond making 3+ CH2 = CH2 + H2 → CH3 - CH3 Nickel catalyst Without catalyst, Ea CH2= CH2 + H2 CH3 - CH3 Surface of catalyst for adsorption With catalyst, Ea adsorption H2 adsorption C2H4 bond breaking making desorption C2H6 Fe2+ catalyst How catalyst work ? Activation energy Effect of diff transition metal on the decomposition of H2O2 measured using a pressure sensor.
- 2. Across period Cr - 4s13d5 • half filled more stable Cu - 4s13d10 • fully filled more stable Ca 4s2 K 4s1 Transition metal have partially fill 3d orbital • 3d and 4s electron can be lost easily • electron fill from 4s first then 3d • electron lost from 4s first then 3d • 3d and 4s energy level close together (similar in energy) Filling electron- 4s level lower, fill first Losing electron- 4s higher, lose first 3d 4s Effect of diff transition metal on the decomposition of H2O2 measured using a pressure sensor.
- 3. Pressure change due to O2 gas Effect of diff transition metal on the decomposition of H2O2 measured using a pressure sensor. Different transition metal were used V5+, Cr3+, Co2+, Ni2+, Mn2+, Pb2+, Cu2+, Fe2+, Fe3+ Same amount were used – 0.00005mol 5% H2O2 used. Pressure sensor to measure O2 released. Reaction mechanism Procedure: 0.00005mol of each catalyst was added to H2O2 Ex: 1g of FeSO4 added to 100ml water – conc is – 0.0359M To transfer 0.00005mol to H2O2, the vol needed will be 1.4ml 1.4ml FeSO4 was added to 1ml 5% H2O2 in a boiling tube Pressure sensor attached. Rxn monitor by increase in pressure 1. Comparing homogenous solution (diff transition metal) against solid MnO2 2. Which transition metal works best (same amt of catalyst added, 0.0005mol) 3. Measure Ea value for diff transition metal and compared to MnO2 which is 50kJmol-1 4. Will Ea higher/lower for heterogenous catalyst (MnO2) compared to homogenous catalyst like CuSO4, FeSO4, FeCI3 Research Questions Hydrogen peroxide decomposition – O2 production 2H2O2→ 2H2O + O2
- 4. Only 4 transition metal works compared to MnO2 Slope/gradient taken over 50s Transition metal Rate kPas-1 V5+ No rxn Cr3+ No rxn Co2+ No rxn Ni2+ No rxn Mn2+ No rxn Pb2+ No rxn Cu2+ 0.009316 Fe2+ 0.03559 Fe3+ 0.1086 MnO2 0.4422 Diff homogenous solution compared to solid MnO2 catalyst. Rate measured as pressure change over time. Effect of diff transition metal on the decomposition of H2O2 measured using a pressure sensor. 0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 0.45 0.5 Cu2+ Fe2+ Fe3+ MnO2 Rate of reaction Rate of reaction vs diff transition metal Homogenous catalyst vs MnO2. Fe3+ works best as and will be chosen for Ea study
- 5. Method 1 Method 2 Time Time Volume Pressure • Rate = Δ vol O2 over time • Volume recorded • Rate = Δ pressure O2 over time • Pressure recorded Procedure 2H2O2 → O2 + 2H2O Rxn: H2O2 with diff (catalyst) measured using TWO diff methods • 2H2O2 → O2 + 2H2O (H2O2 limiting, KI excess) • Pipette 1ml 1.0M KI to 20ml of 1.5% H2O2 • Vol O2 released recorded at 1 min interval • Repeated using 3% H2O2 conc Time/m Vol O2 (H2O2 1.5%) Vol O2 (H2O2 3.0%) 0 0.0 0.0 1 8.5 14.0 2 15.0 26.5 3 21.0 34.0 4 26.0 39.0 Volume O2 Time 3 % 1.5 % Effect of diff transition metal on the decomposition of H2O2 measured using a pressure sensor.
- 6. • 2H2O2 → O2 + 2H2O (H2O2 limiting, KI excess) • Pipette 1ml 1.0M KI to 20ml of 1.5% H2O2 • Pressure O2 released recorded at 1 min interval • Repeat using 3% H2O2 conc Techniques Used to measure Rate of Rxn Method 1 Method 2 Time Time Volume Pressure • Rate = Δ vol O2 over time • Volume recorded • Rate = Δ pressure O2 over time • Pressure recorded Procedure 2H2O2 → O2 + 2H2O Time 3 % 1.5 % Time/m Pressure O2 (H2O2 1.5%) Pressure O2 (H2O2 3%) 0 101.3 101.3 1 102.4 103.4 2 103.5 105.6 3 110.3 115.2 4 113.5 118.2 Pressure O2 Rxn: H2O2 with diff (catalyst) measured using TWO diff methods
