IA on effectiveness of different types of catalysts MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.
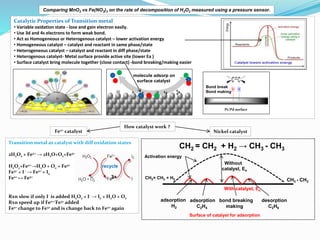
- 1. Pt/Pd surface Catalytic Properties of Transition metal • Variable oxidation state - lose and gain electron easily. • Use 3d and 4s electrons to form weak bond. • Act as Homogeneous or Heterogenous catalyst – lower activation energy • Homogeneous catalyst – catalyst and reactant in same phase/state • Heterogeneous catalyst – catalyst and reactant in diff phase/state • Heterogenous catalyst- Metal surface provide active site (lower Ea ) • Surface catalyst bring molecule together (close contact) -bond breaking/making easier Transition metal as catalyst with diff oxidation states 2H2O2 + Fe2+ → 2H2O+O2+Fe3+ H2O2+Fe2+→H2O + O2 + Fe3+ Fe3+ + I - → Fe2+ + I2 Fe2+ ↔ Fe3+ Rxn slow if only I- is added H2O2 + I- → I2 + H2O + O2 Rxn speed up if Fe2+/Fe3+ added Fe2+ change to Fe3+ and is change back to Fe2+ again recycle molecule adsorp on surface catalyst Pt/Pd surface Bond break Bond making 3+ CH2 = CH2 + H2 → CH3 - CH3 Nickel catalyst Without catalyst, Ea CH2= CH2 + H2 CH3 - CH3 Surface of catalyst for adsorption With catalyst, Ea adsorption H2 adsorption C2H4 bond breaking making desorption C2H6 Fe2+ catalyst How catalyst work ? Activation energy Comparing MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.
- 2. Across period Cr - 4s13d5 • half filled more stable Cu - 4s13d10 • fully filled more stable Ca 4s2 K 4s1 Transition metal have partially fill 3d orbital • 3d and 4s electron can be lost easily • electron fill from 4s first then 3d • electron lost from 4s first then 3d • 3d and 4s energy level close together (similar in energy) Filling electron- 4s level lower, fill first Losing electron- 4s higher, lose first 3d 4s Comparing MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.
- 3. Solid vs solution catalyst will be compared. Assuming same surface area Fe3+ solution and MnO2 solid Same amount were used – 0.00005mol 5% H2O2 used. Pressure sensor to measure O2 released. Diff temp used to find Ea Reaction mechanism Procedure: 0.00005mol of each catalyst added to H2O2 H2O2 and Fe(NO3)3 solution immersed in water bath at various temp. Diff temp used (17, 30, 35, 38, 42C) Mass of MnO2 is 0.00435g – (0.00005mol) Ex: 1g of Fe(NO3)3 added to 100ml water – conc is = 0.0247M To transfer 0.00005mol Fe3+ to H2O2, the vol needed will be 2ml 2ml Fe(NO3)3 was added to 1ml 5% H2O2 in a boiling tube Pressure sensor attached. 1. Comparing homogenous solution (diff transition metal) against solid MnO2 2. Which transition metal works best (same amt of catalyst added, 0.0005mol) 3. Measure Ea value for diff transition metal and compared to MnO2 which is 41kJmol-1 4. Will Ea higher/lower for heterogenous catalyst (MnO2) compared to homogenous catalyst like CuSO4, FeSO4, FeCI3 Research Questions Hydrogen peroxide decomposition – O2 production 2H2O2→ 2H2O + O2 Fe3+ and MnO2 Comparing MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.
- 4. Expt done at diff temp for Fe(NO3)3 Slope/gradient taken over 15s Rate decomposition increases exponential with temp. Temp/ C Temp/ K Rate kPa/s 1/T k ln k 17 290 0.06074 0.00344 0.06074 -2.801 30 303 0.5423 0.00330 0.5423 -0.611 35 308 1.277 0.00324 1.277 0.2445 38 311 1.505 0.00321 1.505 0.409 42 315 2.588 0.00317 2.588 0.951 Assuming rate constant, k. = Rate of decomposition. y = 0.0051e0.1518x R² = 0.989 0 0.5 1 1.5 2 2.5 3 3.5 0 10 20 30 40 50 Rate decomposition temp/C Rate decomposition vs temp/C Comparing MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor. Fe(NO3)3 catalyst at diff temp
- 5. Temp/ C Temp/ K Rate kPa/s 1/T k ln k 17 290 0.06074 0.00344 0.06074 -2.801 30 303 0.5423 0.00330 0.5423 -0.611 35 308 1.277 0.00324 1.277 0.2445 38 311 1.505 0.00321 1.505 0.409 42 315 2.588 0.00317 2.588 0.951 Arrhenius Eqn Ea from its gradient Arrhenius Eqn - Ea by graphical Method RT Ea e A k . . T R E A k a 1 ln ln Plot ln k vs 1/T ln both sides -Ea/R Gradient = -Ea/R Gradient = - 13953 -13953 = -Ea/R Ea = 13953 x 8.314 = 116 kJmol-1 ln k 1/T Arrhenius plot to find Ea for Fe(NO3)3 y = -13953x + 45.294 R² = 0.9913 -3 -2.5 -2 -1.5 -1 -0.5 0 0.5 1 1.5 0.00315 0.0032 0.00325 0.0033 0.00335 0.0034 0.00345 0.0035 lnk 1/T lnk vs 1/T Assuming rate constant, k. = Rate of decomposition.
- 6. Expt done at diff temp for MnO2 Slope/gradient taken over 15s MnO2 catalyst at diff temp. Rate decomposition increases exponential with temp. Temp/ C Temp/ K Rate kPa/s 1/T k ln k 17 290 0.4098 0.00344 0.4098 -0.892 30 303 0.7466 0.00330 0.7466 -0.2922 35 308 0.8274 0.00324 0.8274 -0.1894 38 311 1.353 0.00321 1.353 0.3023 42 315 1.445 0.00317 1.445 0.368 Assuming rate constant, k. = Rate of decomposition. y = 0.1644e0.0514x R² = 0.9426 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 0 10 20 30 40 50 Rate decomposition temp/C Rate decomposition vs temp/C Comparing MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.
- 7. Arrhenius Eqn Ea from its gradient Arrhenius Eqn - Ea by graphical Method RT Ea e A k . . T R E A k a 1 ln ln Plot ln k vs 1/T ln both sides -Ea/R Gradient = -Ea/R Gradient = - 4703 -4703 = -Ea/R Ea = 4703 x 8.314 = 39 kJmol-1 ln k 1/T Arrhenius plot to find Ea for MnO2 Temp/ C Temp/ K Rate kPa/s 1/T k ln k 17 290 0.4098 0.00344 0.4098 -0.892 30 303 0.7466 0.00330 0.7466 -0.2922 35 308 0.8274 0.00324 0.8274 -0.1894 38 311 1.353 0.00321 1.353 0.3023 42 315 1.445 0.00317 1.445 0.368 y = -4703.1x + 15.248 R² = 0.9375 -1 -0.8 -0.6 -0.4 -0.2 0 0.2 0.4 0.6 0.00315 0.0032 0.00325 0.0033 0.00335 0.0034 0.00345 0.0035 lnk 1/T lnk vs 1/T Assuming rate constant, k. = Rate of decomposition. 0 20 40 60 80 100 120 140 Fe(NO3)3 MnO2 Activation energy Type of catalyst Type of catalyst vs activation energy Comparison between solid catalyst (MnO2) vs solution (FeNO3)3 Lit value Ea.for MnO2 = (41 kJmol-1). % 𝐞𝐫𝐫𝐨𝐫 = (𝐋𝐢𝐭 𝐯𝐚𝐥𝐮𝐞−𝐞𝐱𝐩𝐭 𝐯𝐚𝐥𝐮𝐞) 𝐋𝐢𝐭 𝐯𝐚𝐥𝐮𝐞 x 100% % error = 𝟒𝟏−𝟑𝟗 𝟒𝟏 𝐱 𝟏𝟎𝟎% = 𝟓% MnO2 system has a lower Ea compared to Fe(NO3)3 system.
- 8. Method 1 Method 2 Time Time Volume Pressure • Rate = Δ vol O2 over time • Volume recorded • Rate = Δ pressure O2 over time • Pressure recorded Procedure 2H2O2 → O2 + 2H2O Rxn: H2O2 with diff (catalyst) measured using TWO diff methods • 2H2O2 → O2 + 2H2O (H2O2 limiting, KI excess) • Pipette 1ml 1.0M KI to 20ml of 1.5% H2O2 • Vol O2 released recorded at 1 min interval • Repeated using 3% H2O2 conc Time/m Vol O2 (H2O2 1.5%) Vol O2 (H2O2 3.0%) 0 0.0 0.0 1 8.5 14.0 2 15.0 26.5 3 21.0 34.0 4 26.0 39.0 Volume O2 Time 3 % 1.5 % Comparing MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.
- 9. • 2H2O2 → O2 + 2H2O (H2O2 limiting, KI excess) • Pipette 1ml 1.0M KI to 20ml of 1.5% H2O2 • Pressure O2 released recorded at 1 min interval • Repeat using 3% H2O2 conc Method 1 Method 2 Time Time Volume Pressure • Rate = Δ vol O2 over time • Volume recorded • Rate = Δ pressure O2 over time • Pressure recorded Procedure 2H2O2 → O2 + 2H2O Time 3 % 1.5 % Time/m Pressure O2 (H2O2 1.5%) Pressure O2 (H2O2 3%) 0 101.3 101.3 1 102.4 103.4 2 103.5 105.6 3 110.3 115.2 4 113.5 118.2 Pressure O2 Comparing MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.
