Case record...Herpes simplex type 1 encephalitis
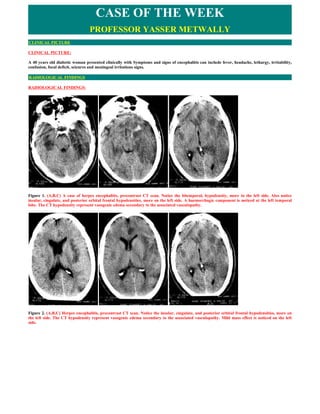
- 1. CASE OF THE WEEK PROFESSOR YASSER METWALLY CLINICAL PICTURE CLINICAL PICTURE: A 40 years old diabetic woman presented clinically with Symptoms and signs of encephalitis can include fever, headache, lethargy, irritability, confusion, focal deficit, seizures and meningeal irritations signs. RADIOLOGICAL FINDINGS RADIOLOGICAL FINDINGS: Figure 1. (A,B,C) A case of herpes encephalitis, precontrast CT scan. Notice the bitemporal, hypodensity, more in the left side. Also notice insular, cingulate, and posterior orbital frontal hypodensities, more on the left side. A haemorrhagic component is noticed at the left temporal lobe. The CT hypodensity represent vasogenic edema secondary to the associated vasculopathy. Figure 2. (A,B,C) Herpes encephalitis, precontrast CT scan. Notice the insular, cingulate, and posterior orbital frontal hypodensities, more on the left side. The CT hypodensity represent vasogenic edema secondary to the associated vasculopathy. Mild mass effect is noticed on the left side.
- 2. Figure 3. (A,B,C) A case of herpes encephalitis, precontrast CT scan. Notice the bitemporal, hypodensity, more in the left side. Also notice insular, cingulate, and posterior orbital frontal hypodensities, more on the left side. A haemorrhagic component is noticed at the left temporal lobe. The CT hypodensity represent vasogenic edema secondary to the associated vasculopathy. CSF examination The opening pressure was elevated, and a CSF pleocytosis was observed. The patients had a mononuclear pleocytosis,(200 cells/mm3 (0.2 × 109 cells/l). CSF protein levels was elevated and the CSF glucose level was normal. Polymerase chain reaction (PCR) was positive for HSV-1 DNA . Detection of HSV-1 DNA in CSF is sensitive and specific, and has become the diagnostic procedure of choice,[1] Electroencephalography Electroencephalography showed background disorganization with generalized and focal slowing, predominantly over the involved left temporal region. Widespread, periodic and stereotyped sharp-wave and slow-wave complexes develop at intervals of 2–3 seconds was seen.[4] DIAGNOSIS: DIAGNOSIS: HERPES SIMPLEX TYPE 1 ENCEPHALITIS DISCUSSION DISCUSSION: There are eight human herpesviruses (HHVs). Primary infection by any of the eight viruses, usually occurring in childhood, is either asymptomatic or produces fever and rash of skin or mucous membranes; other organs might be involved on rare occasions. After primary infection, the virus becomes latent in ganglia or lymphoid tissue. With the exception of HHV-8, which causes Kaposi's sarcoma in patients with AIDS, reactivation of HHVs can produce one or more of the following complications: meningitis, encephalitis, myelitis, vasculopathy, ganglioneuritis, retinal necrosis and optic neuritis. Disease can be monophasic, recurrent or chronic. Infection with each herpesvirus produces distinctive clinical features and imaging abnormalities. This Review highlights the patterns of neurological symptoms and signs, along with the typical imaging abnormalities, produced by each of the HHVs. Optimal virological studies of blood, cerebrospinal fluid and affected tissue for confirmation of diagnosis are discussed; this is particularly important because some HHV infections of the nervous system can be treated successfully with antiviral agents. Herpesviruses are large double-stranded DNA viruses. Approximately 130 different herpesviruses have been identified, not only in mammals, but also in frogs, lizards, birds, fish and mosquitoes. There are eight human herpesviruses (HHVs): herpes simplex virus (HSV-) 1, HSV-2, varicella zoster virus (VZV or HHV-3), Epstein–Barr virus (EBV or HHV-4), cytomegalovirus (CMV or HHV-5), HHV-6, HHV-7, and HHV-8. A characteristic feature of all herpesviruses is their ability to become latent, primarily in ganglia of the nervous system and lymphoid tissue. For example, HSV and VZV become latent in neurons of ganglia, whereas EBV is latent in B lymphocytes. Most of the HHVs are neurotropic and infrequently cause serious acute and chronic neurological disease of the PNS and CNS that might be monophasic, recurrent or chronic. Infection with each herpesvirus produces different clinical features and imaging abnormalities, and many HHV infections can now be treated. This Review focuses on the neurological complications of the HHVs, and discusses optimal virological tests to identify the etiological agent, along with state-of-the-art treatments for these disorders. Herpes Simplex Virus 1 Clinical Features Encephalitis is the most serious neurological complication caused by HSV-1. Symptoms and signs of encephalitis can include fever, headache, lethargy, irritability, confusion, focal deficit, aphasia and seizures, and they reflect virus replication with accompanying inflammation in the medial temporal lobe and orbital surface of the frontal lobe. Progressive temporal lobe edema can lead to uncal herniation, with tachycardia, hyperventilation, flexor (and later extensor) posturing, and a dilated pupil (usually on the side of the herniated temporal lobe). Survivors can be left with a permanent seizure disorder, mental status changes, aphasia or motor deficit. Before the advent of aciclovir treatment, most patients with HSV-1 encephalitis died. Clinical investigations Cerebrospinal fluid The cerebrospinal fluid (CSF) is usually abnormal in patients with HSV-1 encephalitis. The opening pressure is often elevated, and a CSF pleocytosis is observed in over 90% of patients, although its initial absence does not rule out HSV-1 encephalitis. Patients typically have a mononuclear pleocytosis, with a mean of 200 cells/mm3 (0.2 × 109 cells/l). CSF protein levels are usually elevated, but CSF glucose levels are usually normal. Increased CSF antibody to HSV-1, and a reduced serum:CSF antibody ratio, might help to diagnose HSV-1 encephalitis. Antibody titers are not usually detected until 2 weeks or more after disease onset, however, and their practical value lies in retrospective
- 3. presumptive diagnosis. HSV-1 can be isolated from cerebral biopsy or autopsy material, but isolation of the virus from CSF is rare. Polymerase chain reaction (PCR) detection of HSV-1 DNA in CSF is sensitive and specific, and has become the diagnostic procedure of choice,[1] although PCR results can be negative during the early stages of disease.[2] Pathological changes in HSV-1 encephalitis include localized inflammation, hemorrhagic necrosis and formation of inclusion bodies. Herpes Simplex Virus 1 and Bell's Palsy Virologic analysis of endoneurial fluid obtained during decompression surgery revealed HSV-1 DNA in 11 out of 14 patients with Bell's palsy. [23] As seropositivity to HSV-1 is well established by adult life, when Bell's palsy is most common, the palsy probably reflects virus reactivation from latency in the geniculate ganglion[24] rather than primary infection. The mechanism through which the virus damages the facial nerve is unknown. Electroencephalography Electroencephalography in patients with HSV-1 encephalitis shows background disorganization with generalized or focal slowing, predominantly over the involved temporal region.[3] In some patients, widespread, periodic and stereotyped sharp-wave and slow-wave complexes develop at intervals of 2–3 seconds.[4] Bilateral periodic complexes appear if both hemispheres are involved and, although they are seen in other CNS disorders, the presence of such complexes in the setting of fever and rapidly progressive neurological disease is strongly indicative of HSV-1 encephalitis. Neuroimaging CT scanning reveals hypodense lesions involving the medial temporal regions, with a sharp transition from the hypodense temporal lesion to normal basal ganglia.[5] Edema and mass effect occur in 80% of cases, and lesions enhance with contrast. MRI reveals a decrease in T1 signal and an increase in T2 signal in the orbitofrontal and medial temporal lobes and insulae, with abnormalities appearing earlier and more frequently than with CT.[6] With modern neuroimaging techniques, HSV-1 encephalitis is only rarely confused with cerebritis, abscess, tumor or infarction. Figure 1 shows typical CT and MRI changes in patients with HSV-1 encephalitis compared with imaging abnormalities in VZV vasculopathy (see below) and other viral encephalitides. Figure 1. Typical changes seen on CT and MRI in patients with herpesvirus infections. (A,B,C) Herpes simplex virus 1 (HSV-1) encephalitis: T2- weighted MRI brain scan demonstrates bilateral involvement of temporal lobes. The exaggerated signal does not extend beyond the insular cortex (thin arrow), but does involve the cingulate gyrus (thick arrow). Figure 2. MRI study in a patient suffering from herpes encephalitis, A,B,C MRI T1 precontrast, D,E MRI T2, F,G,H,I are FLAIR MRI images.
- 4. Notice the T1 hypointensity predominantly involving the bitemporal cortex more on the right side, the insular, the occipital orbital frontal (F) and the cingulate cortex, again more on the right side. The T1 involved zones are hyperintense on the T2 and FLAIR studies. Notice that the encephalitic process is predominately cortical (A,B,C). Temporal lobes involvement is more medially than laterally. The MRI signal changes are predominantly due to vasogenic edema, the signal intensity of edema is different from the CSF in flair studies. Pathogenesis and Latency Primary HSV-1 infection often results in painful skin or mucosal lesions, but can also be asymptomatic. Virus replicates at the portal of entry, usually oral or genital mucosal tissue, leading to infection of sensory nerve endings. Virus is then transported to regional ganglia where it establishes latency. Recurrence is directly related to the severity of primary infection, as reflected by the size, number and spread of lesions. The mechanism by which HSV-1 infects the CNS to cause encephalitis has not been definitively established. The predilection of HSV-1 for the orbital surface of the frontal lobe and medial surface of the temporal lobe indicates that virus might spread from olfactory mucosa through the cribiform plate of the ethmoid bone into the anterior fossa. Latent virus in the trigeminal ganglia might also reactivate and spread via tentorial nerves that innervate the meninges of the anterior and middle cranial fossa. Figure 3. A, This is a gross photograph of a section of brain showing multiple small, punctate hemorrhages throughout the brain parenchyma, especially the insula and the medial temporal cortex, in a case of herpes encephalitis. B, A case of herpes encephalitis with gross haemorrhagic necrosis of the temporal lobe. HSV-1 latency is restricted to cranial nerve ganglia, as indicated by spontaneous, recurrent outbreaks of vesicles on the mouth, or by isolation of HSV-1 from postmortem explants of human trigeminal,[7,8] nodose, vagal[9] and ciliary[10] ganglia. HSV-1 DNA sequences have been detected in human thoracic ganglia[11] and brain,[12] but virus has never been recovered from these sites. Most humans harbor latent HSV-1.[11] Neurons are the exclusive site of HSV-1 latency in human ganglia, and also in mouse and rabbit models. Studies of latently infected mouse ganglia have revealed HSV-1 DNA in 1–30% of neurons.[13] In humans, the latent HSV-1 DNA copy number ranges from less than 300 copies/105 cells to over 10,000 copies/105 cells in trigeminal ganglia,[14] and has a mean of 176,705 ± 255,916 copies/105 cells in vestibular ganglia, 9,948 ± 22,066 copies/105 cells in geniculate ganglia, and 3,527 ± 9,360 copies/105 cells in cochlear ganglia.[15] During latency, HSV-1 DNA is endless—that is, circular or concatameric[16]—and does not integrate into the host genome. Figure 4. Right, Coronal section showing mesial temporal lobe, insular necrosis in a case of herpes simplex virus (HSV) encephalitis. Left, Typical homogenous, eosinophilic Cowdry A inclusion in HSV encephalitis. In ganglia latently infected with HSV-1, only one region of the viral genome is abundantly transcribed. This region encodes the latency- associated transcripts (LATs). The LAT domain is transcriptionally complex, and although the predominant species that accumulates in the neuronal nucleus during latency is a 2.0 kb stable intron,[17] other RNA species are also transcribed, including a number of lytic or acute-phase transcripts. No LAT protein has ever been detected. LAT-deficient HSV-1 mutants become latent,[18] and splicing of the primary LAT transcript appears to be important in promoting latency.[19] The function of LATs is unknown, but they might protect neurons from apoptosis, [20] perhaps by acting through microsomal RNA.[21]
- 5. Figure 5. A precontrast CT scan showing a case of herpes encephalitis, notice the left medial temporal hemorrhagic zone surrounded by edema. Figure 6. Section at the level of the anterior commissure showing softening and disruption of the right lateral temporal lobe with some hemorrhage in a case of herpes encephalitis HSV-1 reactivates after ultraviolet (UV) light stimulation, epinephrine iontophoresis, hyperthermia, or even social stress. Variables include duration of stimuli and latent virus DNA burden. Accumulating evidence from rodent models indicates that HSV-1 infection suppresses the hypothalamic–pituitary–adrenal axis during primary infection, and that this stress-induced suppression has a role in virus reactivation. The relevance of animal models to humans must, however, always be questioned. For example, guinea pigs vaccinated with HSV-2 glycoprotein D were protected from reactivation, whereas subunit vaccines were only marginally effective in human trials.[22] Figure 7. Schematic of the typical areas of involvement by herpes simplex virus. Note the propensity of involvement in the medial temporal lobes, in the insular cortex and the cingulate cortex. Treatment Intravenous aciclovir (10 mg/kg body weight three times a day for 14–21 days) is the standard treatment for HSV-1 encephalitis in adults. Steroids are sometimes given as well, although data regarding the efficacy of this approach remain anecdotal. Cognitive impairment and seizures are significant neurological sequelae in treated patients. Acyclovir, an acyclic analogue of guanosine, is the antiviral drug of choice for treatment of neurologic disease associated with herpes simplex virus-1 or herpes simplex virus-2 infection. Its mechanism of action depends on a virus-specified thymidine kinase that phosphorylates acyclovir to its monophosphate derivative. Acyclovir monophosphate is phosphorylated further by cellular kinases to acyclovir triphosphate, which binds to virus- induced DNA polymerase, acting as a DNA chain terminator. Because acyclovir is taken up selectively by virus-infected cells, the concentration of acyclovir triphosphate is 40 to 100 times higher in infected cells than in uninfected cells. In addition, virus-induced DNA polymerase exhibits a 10- to 30-fold greater affinity for acyclovir triphosphate than do cellular polymerases. For these reasons, the drug exhibits low toxicity for uninfected cells and is therefore well tolerated clinically. Acyclovir is highly specific for the herpes virus and a clinical response to acyclovir has also a diagnostic implications as its action depends on a virus-specified thymidine kinase. No clinical response will occur if the offending organism is other than the herpes virus. The relative safety of acyclovir has led to the common practice of presumptive treatment of patients who exhibit typical clinical signs and symptoms of the disease with typical radiological findings. The outcome of acyclovir therapy depends on the age of the patient and level of consciousness when treatment is initiated.
- 6. Massive brain edema is observed in most of patients. Corticosteroids are probably not useful in the treatment of edema associated with HSE. Data from animal studies have shown even potentiation of disease. 48 Brain edema in all patients responded dramatically, even when massive, to acyclovir It seems quite logic that treatment of the cause of brain edema is the best treatment of brain edema. The goals of using antivirals are to shorten the clinical course, prevent complications, prevent development of latency and subsequent recurrences, decrease transmission, and eliminate established latency. Table 1. Acyclovir therapy Acyclovir (Zovirax)- Has demonstrated inhibitory activity against both HSV-1 and HSV-2 and is taken up selectively by infected cells; rate of mortality from Drug Name HSE before use of acyclovir was 60-70%—since acyclovir, it is approximately 30%. Adult Dose 10 mg/kg/dose IV or 500 mg/m2/dose IV q8h Pediatric Dose Administer as in adults Contraindications Documented hypersensitivity Half-life prolonged and toxicity increased by concomitant probenecid or Interactions zidovudine Pregnancy B - Usually safe but benefits must outweigh the risks. Use caution in patients with renal failure or receiving other nephrotoxic drugs Precautions concurrently Figure 8. A case of herpes encephalitis treated with acyclovir, notice the massive brain oedema before treatment (A), also notice the gradual reduction of brain oedema in (B) and complete resolution of brain oedema in (C) following treatment with acyclovir, this was coupled with dramatic clinical improvement. Notice the residual frontal infarction that became apparent after resolution of the encephalitic process. Table 2. Clinical Features of Virus Reactivation Feature Herpes simplex Varicella zoster Reactivation Multiple times Once age Young Elderly Rash Localized Dermatomal Aetiology Sunlight, trauma Immunosuppression Herpes Simplex Virus 2 Clinical Features HSV-2 causes genital herpes, and neurological complications include aseptic meningitis and recurrent radiculopathy. HSV-2 also causes myelitis in immunocompromised individuals. Typical features of HSV-2 meningitis are headache, fever, stiff neck, and lymphocytic pleocytosis in the CSF. HSV-2 meningitis might be preceded by pelvic inflammatory disease, genital pain or both, and clinical examination should include a search for vesicular lesions over the external genitalia, and also for lesions in the vagina or on the cervix. It is important to recognize, however, that many patients with HSV-2 meningitis do not have active genital lesions at the time of meningitis, and often have no known history of genital herpes. HSV-2 was shown to cause aseptic meningitis in one patient with recurrent dermatomal distribution skin lesions.[25] PCR has shown that HSV-2,[26] and less often HSV-1,[27] causes benign recurrent lymphocytic meningitis, which is usually self-limited. HSV-2 encephalitis occurs most often in newborn babies and, more rarely, in immunocompromised adults. Approximately 5% of HSV encephalitis cases in immunocompetent adults are also attributable to HSV-2. In infants and immunocompromised adults, HSV-2 encephalitis is diffuse, unlike the focal lesions produced by HSV-1 infection. Nevertheless, seizures, alterations in the state of consciousness, and focal neurological deficits also characterize HSV-2 encephalitis. HSV-2 and CMV encephalitis can coexist in patients with AIDS. The clinical features of HSV-2 encephalitis in immunocompetent adults are generally similar to those seen with HSV-1 encephalitis, although some immunocompetent patients with HSV-2 encephalitis have less-aggressive forms of encephalitis. HSV Neuropathy (Zosteriform Eruption) HSV-2 can produce pain and dermatomal distribution rash (zosteriform eruption). Herpes lesions above the neck typically result from reactivation of HSV-1, whereas lesions below the waist result from reactivation of HSV-2, although exceptions to this rule occur. There is usually a prodrome of diffuse neuralgia, often with malaise and fever, followed within days by the appearance of vesicles.[29] Most reports describe lesions in one or more of the facial areas served by the trigeminal nerve, and also on the trunk, extremities and genitalia. Recurrent sciatica has
- 7. been associated with HSV.[30] A first episode can be confused with herpes zoster, but recurrent episodes of dermatomal neuralgic pain and zosteriform eruptions are usually caused by HSV-2.[25] Although HSV neuropathy is now well documented, the exact type of HSV responsible for each form of neuropathy is still unknown. Future DNA analysis of herpesvirus isolates, by PCR with virus-specific primers, should clarify whether neuropathy is caused primarily by HSV-1 or HSV-2. HSV Meningitis, Myelitis and Brainstem Encephalitis HSV meningitis is usually caused by HSV-2. There have been no controlled trials of antiviral therapy for either isolated or recurrent HSV meningitis, although noncontrolled experience indicates that treatment with aciclovir or related antiviral drugs might reduce the duration and severity of attacks. Prophylactic antiviral treatment might also reduce the frequency and severity of recurrent meningitis, although its therapeutic efficacy has not been clearly established. HSV-2 can also cause monophasic or recurrent brainstem encephalitis and myelitis. As almost all published studies are single-case reports, it is not possible to evaluate the efficacy of antiviral therapy for these conditions. The severity of these infections has, however, highlighted the need for treatments, usually the same as those for HSV encephalitis. In patients with recurrent disease, valaciclovir or aciclovir have been used to reduce the likelihood of recurrences. Pathogenesis and Latency HSV-2 has been isolated from normal human sacral ganglia,[28] but the physical state of HSV-2 DNA and HSV-2 gene expression in latently infected human ganglia have not been studied nearly as extensively as the characteristics of HSV-1 in trigeminal ganglia. Latent lumbosacral ganglionic infection can be established in mice and guinea pigs by intravaginal infection, and virus can be reactivated after sciatic nerve section (mice) or UV irradiation (guinea pigs). Whereas HSV-1 latency is found only in cranial nerve ganglia, HSV-2 becomes latent in lumbrosacral ganglia. Treatment The treatment for HSV-2 encephalitis is the same as for HSV-1. Varicella Zoster Virus (Herpes Simplex Virus 3 or HHV-3) Clinical Features VZV causes chickenpox (varicella), after which the virus becomes latent in cranial nerve, dorsal root and autonomic nervous system ganglia along the entire neuraxis. Decades later, a declining VZV-specific host immunity[31] leads to virus reactivation, usually resulting in herpes zoster (also known as shingles), which is characterized by pain and rash restricted to 1–3 dermatomes. The NIH Shingles Prevention Study estimates that herpes zoster affects between 600,000 and 1,000,000 Americans each year.[32] The incidence and severity of herpes zoster in immunodeficient individuals can be viewed as a continuum, ranging from a natural decline in VZV-specific immunity with age, to more serious host immune deficits such as those encountered in transplant recipients, or in patients with cancer or AIDS. Because VZV is latent in most ganglia, herpes zoster can occur anywhere on the body. The most common sites are thoracic, and in the cutaneous distribution of the ophthalmic branch of the trigeminal nerve. VZV reactivation from the geniculate (seventh cranial nerve) ganglion can cause the Ramsay Hunt syndrome, features of which include peripheral facial weakness and rash around the ear (zoster oticus). In many herpes zoster patients over the age of 60 years, pain—so-called postherpetic neuralgia (PHN)—continues for months or years. Although the cause of PHN is uncertain, analyses of blood, CSF and postmortem ganglia indicate that it is related to persistent or low-grade productive infection in ganglia.[33] After herpes zoster, VZV can also spread to blood vessels of the brain, producing a unifocal or multifocal vasculopathy, particularly in immunocompromised individuals. Virological analyses of patients who died from VZV vasculopathy have revealed both VZV DNA and VZV antigen in cerebral arteries,[34] indicating active virus infection. Unifocal vasculopathy, formerly called granulomatous arteritis, is the predominant form in elderly immunocompetent adults, and is characterized by an acute focal deficit that develops weeks to months after contralateral trigeminal distribution herpes zoster. Multifocal vasculopathy develops in immunocompromised individuals, and is associated with headache, fever, mental status changes and focal deficit. CSF mononuclear pleocytosis is usually present in both vasculopathies. Brain MRI scanning reveals a single large infarct in unifocal vasculopathy; in multifocal vasculopathy, ischemic and hemorrhagic infarcts are seen in both cortical and subcortical areas (Figure 1). Cerebral angiography reveals focal arterial stenosis. Pathological changes in affected arteries include the presence of multinucleated giant cells, Cowdry A inclusion bodies and herpesvirus particles. Virological analysis reveals VZV DNA and antigen in affected vessels. Vasculopathy occurs without rash, can recur, and presents with transient ischemic attacks, including posterior ischemic optic neuropathy, remote from, and months after, acute herpes zoster.[35] Detection of VZV DNA or antibody in CSF confirms a diagnosis of VZV vasculopathy. As infection is active and often protracted, anti-VZV IgM or IgG antibody is found in CSF, with reduced serum:CSF ratios of VZV antibody compared with total IgG or albumin.[36] Detection of antibody to VZV in CSF, even without amplifiable VZV DNA, can be diagnostic. Recognition of the wide spectrum of VZV vasculopathy and its proclivity to recur is essential, as effective antiviral therapy can be curative. Pathogenesis and Latency The dermatomal distribution of herpes zoster lesions, combined with peak VZV titers in varicella or herpes zoster vesicles, indicate that virus that has reactivated from latency in PNS ganglia spreads to the skin by intra-axonal transportation, although the presence of VZV in multiple organs in disseminated zoster is most consistent with hematogenous dissemination. VZV-associated viremia and lymphotropism have been documented during varicella, herpes zoster, PHN and zoster sine herpete, and even in normal healthy adults. During varicella, T cells are productively infected, probably contributing to the spread of the virus. VZV DNA, transcripts and protein have been detected in mononuclear cells (MNCs) of herpes zoster and PHN patients. With one exception,[37] attempts to recover infectious VZV from MNCs of immunocompetent herpes zoster patients have failed.[38,39] In herpes zoster and PHN patients, lack of detectable infectious virus might be attributable to the cell- associated nature of the virus, low-level or abortive infection of MNCs, or a decrease in the frequency of MNCs that support productive infection, as compared with primary infection. The detection of VZV DNA[40,41] and VZV-specific proteins[42] in blood MNCs of some patients with PHN and zoster sine herpete,[43,44,45] as well as in tissue of patients with chronic VZV ganglionitis,[46] might reflect low-level productive infection in ganglia—a hypothesis that is supported by favorable clinical responses in some PHN patients treated with antiviral agents.[47,48] MNCs, and in particular antigen-presenting
- 8. cells, might acquire virus while trafficking through ganglia. Ultimately, digestion of virus would account for the detection of some, but not all, regions of the VZV genome in circulating MNCs,[49] compared with the presence of the entire virus genome in latently infected ganglia. Digestion of viral DNA by MNCs also helps to explain why VZV DNA is found in MNCs from only 20% of patients with PHN; there would only be a limited probability that any given fragment of viral DNA would be amplified by PCR. Unlike HSV-1, VZV cannot be isolated from latently infected human ganglia, although nucleic acid hybridization and PCR have detected VZV DNA in ganglia during latency.[11,50] Whereas HSV-1 latency is mostly restricted to cranial nerve ganglia, VZV is latent in cranial nerve, dorsal root and autonomic nervous system ganglia in over 90% of healthy adults. As in the case of HSV-1, neurons are the exclusive site of latent VZV. [51,52] Analysis of trigeminal ganglia revealed no difference in VZV copy number between the left and right trigeminal ganglia of the same individual, but showed a wide variation of 19–3,145 copies per latently infected ganglion between individuals,[14] probably reflecting the uneven amount of VZV encountered during primary infection. Like HSV-1, VZV DNA exists as an endless (either circular or concatameric) episome. [53] Five VZV genes corresponding to open reading frames 21, 29, 62, 63 and 66 are transcribed, and proteins from three of these (62, 63 and 66) have been found in latently infected ganglionic neurons. Important differences between the clinical features of HSV and VZV reactivation are cited in Table 2 . Treatment As the neurological complications of VZV infection are generally caused by replication of VZV in the nervous system, inhibition of replication is an obvious target for treatment. Herpes Zoster Antiviral therapy is recommended for immunocompetent adults over 50 years of age with herpes zoster, particularly if it can be initiated within 72 hours of lesion onset. The improved pharmacokinetic profiles and simpler dosing regimens of oral valaciclovir (1,000 mg three times daily for 7 days) or famciclovir (250 mg or 500 mg three times daily) have led to their use instead of aciclovir (800 mg five times daily) as the preferred treatment. Steroids might reduce the inflammation that contributes to acute pain, provided that there are no contraindications and the potential risk of excessive adverse effects is explained. Although they do not prevent PHN, steroids reduce acute symptoms and might facilitate a return to normal quality of life. There is no indication for the use of topical antiviral agents to treat cutaneous herpes zoster. The premise that the pain experienced by people with herpes zoster is primarily attributable to inflammation and necrosis of neurons led to the suggestion that, by reducing inflammation, corticosteroids would reduce pain. The debate on the role of corticosteroid therapy in herpes zoster has largely been resolved by two large prospective clinical trials, which demonstrated the benefit of corticosteroids in reducing the duration of acute pain, although neither study showed any reduction in the incidence or duration of PHN among corticosteroid recipients.[54,55] Postherpetic Neuralgia PHN is operationally defined as pain that persists for more than 3 months after herpes zoster. Tricyclic antidepressants, such as amitriptyline or nortriptyline (initiated at 10–25 mg and increasing weekly by 25 mg [10 mg if the patient is >65 years old or frail]), relieve pain in some patients. Gabapentin, 300–900 mg/day up to 3,600 mg/day (provided that there is unimpaired renal function), is also recommended. Pregabalin is another FDA-approved drug that is being used increasingly to treat PHN. A topical local anesthetic (e.g. lidocaine patch 5%) might also help. Vasculopathy and Myelitis Vasculopathy and myelitis are treated with intravenous aciclovir (10–15 mg/kg every 8 hours for 10–14 days). The role of antiviral therapy in individuals presenting with rarer complications of varicella, such as cerebellar ataxia, has not been studied in a prospective or controlled fashion; however, administration of intravenous aciclovir to such patients is likely to be appropriate. Immunocompromised patients might require longer periods of treatment. Treatment should be discontinued if both VZV DNA and anti-VZV antibody are absent from CSF collected at the time of treatment initiation. Epstein–Barr Virus (Herpes Simplex Virus 4 or HHV-4) Clinical Features EBV causes aseptic meningitis, encephalomyeloneuritis and neuritis. The EBV neuropathies present with ophthalmoplegia, lumbosacral plexopathy, and sensory or autonomic neuropathy. Historically, the association of virus with neurological disease was suggested by a positive serum heterophile antibody titer, and later by the presence of EBV DNA, antibody or both in CSF.[56] EBV is latent in B cells, and caution should be used in attributing neurological disease to EBV purely on the basis of a positive CSF PCR; in previously EBV-infected individuals, PCR might detect EBV DNA latent in B cells that are part of the inflammatory response induced by another agent. The presence of anti-EBV IgM or IgG antibody in CSF is more likely to be significant, particularly if there is evidence of intrathecal synthesis of EBV antibody (see below). In a comprehensive clinical, radiological and virological analysis of four patients with EBV myeloradiculitis, encephalomyeloradiculitis and meningoencephalomyeloradiculitis,[57] the CSF contained a mononuclear pleocytosis with elevated protein and normal glucose; in two patients, MRI scans revealed an increased signal in the spinal cord and lumbosacral roots, but no brain swelling or focal changes. In all four patients, EBV DNA was found in the CSF and there were reduced serum:CSF ratios of antibody to EBV, but not to total IgG or albumin, consistent with intrathecal antibody synthesis. Residual neurological deficits were evident. Disappearance of viral DNA from CSF at the time of improvement of neurological disease, particularly before a decline in CSF white blood cells, supports the theory that CNS disease results from direct invasion of the CNS by virus. EBV DNA has been found in brain biopsies from affected patients.[58] CSF from patients with EBV infection of the nervous system contains mainly EBV-specific CD8+ T cells, a few CD4+ T cells, and no CD19+ B cells.[59] CD8+ cells from one patient with EBV infection were shown to contain EBV DNA, and had unrestricted cytotoxic activity.[60] Patients with postinfectious complications of EBV infection have a low EBV load and a high CSF leukocyte count. Encephalitis is characterized by intense viral replication and vigorous inflammation, whereas CNS lymphoma is associated with a limited inflammatory response. EBV-associated CNS lymphomas are rare in immunocompetent individuals. EBV triggers AIDS-associated primary CNS lymphoma (PCNSL) and post-transplantation lymphoproliferative disorder (PTLD). EBV DNA can be found in tumor tissue in both of these conditions[61] and in CSF of patients with AIDS-associated PCNSL. Deficiency in cytotoxic T lymphocytes seems to permit the outgrowth of EBV-transformed B cells, resulting in PTLD. Administration of autologous EBV-specific cytotoxic T cells has been shown to produce specific killing of EBV-transformed B cells in patients with PTLD.[62] Pathogenesis and Latency
- 9. EBV infects B and T lymphocytes of more than 90% of the general population before adulthood. Primary infection results in transient viremia, followed by a rapid immune response. EBV replicates in the oropharynx and is transmitted through oral secretions. Primary EBV infection frequently results in infectious mononucleosis. EBV is also associated with nasopharyngeal carcinoma, Burkitt's lymphoma, Hodgkin's disease, and lymphoproliferative disease in immunocompromised individuals.[63] It is estimated that neurological complications occur in 1–5% of individuals with infectious mononucleosis. Given the widespread prevalence of EBV infection, the burden of neurological disease is probably underestimated; for example, virtually all CNS lymphomas in AIDS patients contain EBV DNA.[61] EBV establishes latency in B lymphocytes, which adopt restricted patterns of EBV gene expression. EBV latency has been classified into three types: type I (Burkitt's lymphoma), in which only the EBV-encoded nuclear antigen (EBNA-1) is expressed; type II (nasopharyngeal carcinoma), in which EBNA-1 is coexpressed along with the latent membrane proteins LMP-1, LMP-2A and LMP-2B; and type III (lymphoproliferative diseases in immunosuppressed individuals), in which five EBNAs and the three LMPs are expressed. Autoactivation of gene expression by LMP- 1 might be important in type II latency, and EBNA-2-dependent regulation of LMP-1 expression appears to be important in type III latency.[64] The precise mechanism by which EBV reactivates remains unknown, however. Treatment There is no known effective antiviral or other accepted treatment for EBV. Cytomegalovirus (Herpes Simplex Virus 5 or HHV-5) Clinical Features CMV produces neurological disease predominantly in infants with congenital infection. Although most congenital CMV infections are asymptomatic, many carriers develop sensorineural hearing loss and intellectual handicaps. Other neurological complications include microcephaly, seizures, hypotonia and spasticity. In immunocompetent adults, the most common neurological complication of CMV infection is Guillain–Barré syndrome, and CMV has also been associated with acute brachial plexopathy.[65] The natural history of CMV infection of the CNS in immunocompetent adults is unknown. CMV encephalitis was first described in renal[66] and bone marrow transplant recipients, and CMV infections in patients with AIDS can cause encephalitis, myelitis, retinitis and polyradiculitis. The CSF might show neutrophilic or mononuclear pleocytosis, or an elevated protein and depressed glucose content, and MRI can reveal enhancement in the ventricular ependyma. Focal disease has been attributed to CMV vasculitis or demyelination. Characteristic owl-eyed cytomegalic inclusions and CMV-specific antigens have been found in brain tissue and blood vessels of AIDS patients with subacute encephalopathy. Complicating the picture, however, is the additional presence of HSV-2. It is difficult, therefore, to attribute specific symptoms and signs to CMV. CMV polyradiculopathy in patients with AIDS begins insidiously as a cauda equina syndrome with paresthesias and distal weakness (usually asymmetric), incontinence, and sacral-distribution sensory loss. CSF pleocytosis is usually present, often with a predominance of polymorphonuclear leukocytes. Most cases have elevated CSF protein and hypoglycorrhachia. CMV can be isolated from CSF, and CMV DNA can be amplified by CSF PCR. Postmortem examination reveals inflammation, necrosis and focal vasculitis of nerve roots, along with typical CMV inclusions.[67] Pathogenesis and Latency CMV has a genome nearly twice as large as those of other HHVs, one which encodes more than 200 genes. During primary infection, CMV replicates in leukocytes and vascular endothelial cells, after which virus becomes latent principally in bone marrow progenitor cells and myeloid cells.[68] Human CMVs show DNA sequence variability[69] and, when adapted to in vitro growth, acquire extensive DNA deletions.[70] Reactivation and shedding of infectious human CMV follows immunosuppression, differentiation of latently infected progenitor cells, or allogeneic organ transplant.[71] After reactivation, CMV eludes immunological clearance by suppressing MHC class I and class II responses, inhibiting apoptotic cell death, and sequestering chemokines.[72] Treatment There have been no randomized controlled studies of antiviral therapy in adults with CMV infection of the nervous system. An international panel that developed guidelines for treatment of CMV diseases in adults with AIDS receiving highly active antiretroviral therapy[73] recommended treatment with intravenous ganciclovir, intravenous foscarnet, or a combination of the two drugs. Patients who develop CMV polyradiculopathy while on ganciclovir or foscarnet therapy should receive combination therapy with both drugs. Patients not on treatment at the time of disease onset could be treated with either drug as monotherapy, and switched to combination therapy if they progressed clinically and/or had persisting CSF pleocytosis (a potential marker of drug failure). Reports of antiviral therapy for CMV-associated mononeuritis multiplex or painful symmetrical neuropathy are too limited to permit conclusions about efficacy of antiviral therapy. Human Herpesvirus 6 Clinical Features HHV-6 causes roseola, a common childhood exanthematous disease. Neurological complications can result from direct invasion of the CNS. HHV-6 DNA has been detected in the CSF of patients with febrile seizures[74] and in the brains of patients with fulminant hepatitis.[75] HHV-6 antigen was found in brain tissue from a fatal case of roseola.[76] HHV-6 causes encephalitis primarily after bone marrow or stem cell transplantation,[77] and might present as limbic encephalitis.[78] Diagnosis is confirmed by detection of HHV-6 DNA in the CSF. On the basis of the presence of HHV-6 DNA and increased levels of antibody to HHV-6 in blood and CSF of patients with multiple sclerosis (MS), HHV-6 has been implicated in the pathogenesis of the disease. HHV-6 is, however, found in only a minority of MS patients, and HHV-6 DNA and elevated HHV-6 antibody levels are also seen in patients with other neurological diseases. Human herpesvirus-6 infection, febrile seizures, and encephalitis Another recent important development has been the identification of human herpesvirus-6 (HHV-6) and the elucidation of its role in human disease. HHV-6, a member of the Herpesviridae family of viruses, was discovered in 1986. Although biologically similar to cytomegalovirus (CMV), the virus was recognized as serologically and genetically distinct from other human herpes viruses [89]. Soon thereafter, HHV-6 was noted to be ubiquitous and a principal cause of the common childhood disease exanthem subitum (also referred to as roseola or sixth disease) [89]. From the neurologic perspective, HHV-6 is of importance because of its linkage to febrile seizures and encephalitis.
- 10. Exanthem subitum typically occurs between 6 months and 2 years of age and is characterized by an abrupt rise in temperature, often to approximately 40°C, followed several days later by a rapid defervescence that coincides with the emergence of an erythematous maculopapular rash that can persist for several days. Fever is a prominent component of the disease, and it has been recognized for many years that febrile seizures occur commonly during the early (febrile) stage of exanthem subitum. The linkage of exanthem subitum to febrile seizures, along with the identification of HHV-6 as the causative pathogen for exanthem subitum, led naturally to the hypothesis that HHV-6 infection may underlie many cases of febrile seizures. This has proven to be true. Multiple studies have demonstrated that a substantial proportion of febrile seizures occur in children with a primary HHV-6 infection [89]. The risk of febrile seizures is high during primary infection with HHV-6, whether or not the child develops the rash of exanthem subitum. In addition to increasing the risk of febrile seizures, a primary infection with HHV-6 may increase the severity of the febrile convulsion. Partial seizures, prolonged seizures, and repeated seizures are more common when the febrile seizures are associated with HHV-6 infection than when the seizures are caused by other pathogens [89]. Furthermore, reports have suggested that HHV-6 may persist within the CNS [89] and that reactivation of HHV-6 may be associated with recurrent febrile seizures [89]. This remains controversial, however, and at least one study has found that children with an initial febrile seizure induced by HHV-6 infection have a lower risk of febrile seizure recurrence than do children whose febrile seizures were triggered by other pathogens [89]. Although the association of HHV-6 infection with febrile seizures is well documented, the mechanism underlying this association remains unclear. One possibility is that HHV-6 directly invades brain parenchyma during a primary infection and that the febrile convulsion is triggered by brain infection. This hypothesis is supported by the findings that HHV-6 is a neurotropic virus that can replicate in glial cells and in brain endothelial cells [89]. Identification of HHV-6 DNA in CSF of patients with febrile seizures further supports the notion that the brain is invaded during HHV-6 infection [89]. Other studies have not found HHV-6 DNA in the CSF of patients with febrile seizures [89], however, and the question of brain invasion in typical cases of HHV-6 infection remains controversial. A second possibility is that the seizures are triggered not by direct viral invasion of the CNS but by the production of a toxin or inflammatory molecule liberated as part of the viral infection. Cytokines, for example, are produced at high levels by circulating monocytes during HHV-6 infection and can act on neurons to lower the seizure threshold [89]. A third possibility is that the febrile convulsions are not directly related to the HHV-6 infection per se but to a characteristic of the fever induced by the infection. Febrile seizures occur almost exclusively in children, presumably because the immature brain has a lower seizure threshold to the destabilizing effects of temperature elevation. A hallmark of exanthem subitum is a rapid onset of high fever. Thus, children infected with HHV-6 may be at greater risk for seizures than children with other infections because of the rapidity of high fever onset with HHV-6. Arguing against this possibility, however, are the findings that neither the maximal temperature nor the duration of fever before the seizure differed between febrile children who developed seizures and those who did not [2,16]. In a few patients, HHV-6 produces encephalitis. Encephalitis caused by HHV-6 has been observed both in immunocompromised patients and in children whose immune function was apparently normal [89]. As might have been anticipated from the association of HHV-6 with convulsions, seizures, and status epilepticus are common presenting signs of HHV-6 encephalitis [89]. Confusion and headache, common symptoms of encephalitis in general, are also commonly observed in encephalitis caused by HHV-6. When a patient develops encephalitis in association with exanthem subitum, the signs of encephalitis usually arise during the febrile phase of the illness before the onset of rash [89]. Whether invasion of the brain by HHV-6 is necessary to produce encephalitis is unresolved. In some cases of HHV-6 encephalitis, viral antigen has been detected within brain astrocytes and neurons [89], the location of viral antigen has been correlated with the sites of neuropathologic changes [19], and HHV-6 DNA has been detected in CSF. These findings strongly suggest that viral invasion occurs and that the presence HHV-6 within the brain plays a key role in the pathophysiology. Nevertheless, other studies have noted the distinct absence of viral antigen from the brain [21] and of viral DNA from the CSF [19] in cases of HHV-6 encephalitis. These results suggest that in at least some cases of HHV-6 encephalitis, the brain dysfunction may be caused by an extrainfectious process. Most children who develop HHV-6 encephalitis have a good outcome with no neurologic sequelae. Some children are left with serious permanent neurologic problems, however, including mental retardation, hemiplegia, and epilepsy [89]. Occasional cases of HHV-6 encephalitis have been fatal [89]. Figure 9. Schematic representation of HHV-6 and HHV-7 genomes. The genomes are colinear. Homologies are 46.6% to 84.9%. Red blocks represent the herpesvirus core genes, numbered from I to VII. Yellow blocks represent ß-herpesvirus subfamily-specific genes (from U2 to U14). Green blocks indicate genes present only in the Roseolovirus genus, i.e., in HHV-6 and HHV-7. Only three ORFs (U22, U83 and U94) are present in HHV-6 and absent from HHV-7 Pathogenesis and Latency As HHV-6 becomes latent in cells of the monocyte–macrophage lineage,[79] the detection of HHV-6 DNA and antigen in brain tissue is likely to reflect HHV-6 reactivation from latency in blood MNCs trafficking through the brain in patients with inflammatory CNS disease. CD46 is an HHV-6 receptor that is expressed mostly in macrophages and cells lining blood vessels, and less often in cells of neuronal origin. HHV-6 expresses proinflammatory cytokines that are cytotoxic to oligodendrocytes and induce caspase-independent apoptosis.[80] HHV-6 has also been shown to inhibit G1–S-phase transition in glial precursor cells, which are important in remyelination.[81] Reactivation of latent HHV-6 is frequently associated with drug-induced hypersensitivity syndrome, possibly because of the acute decrease in serum IgG levels observed in these patients. Treatment
- 11. There have been no randomized controlled trials of antiviral therapies in either immunocompetent or immunocompromised patients. Ganciclovir and foscarnet have been used, but their efficacy is unknown. The outcome in immunocompetent patients does not appear to be substantially better than that in immunocompromised individuals. Real-time fluorescent probe CSF PCR has been used to follow the effects of antiviral therapy on HHV-6 DNA levels in the CSF of 11 hematopoietic stem cell recipients with HHV-6 infection (8 of whom had HHV-6 encephalitis).[82] All patients were treated with ganciclovir, foscarnet or both. Within 3 weeks of commencing antiviral therapy, median CSF viral load had decreased from 25,000 copies/ml to 100 copies/ml, although the difference was not statistically significant (P = 0.13). Four of the eight patients with HHV-6 encephalitis improved clinically, with viral load declining concurrently with antiviral therapy. Human Herpesvirus 7 HHV-7 is closely related to HHV-6, and can induce reactivation of the latter in vitro. HHV-7 was first isolated from CD4+ T cells of a healthy 26- year-old under conditions that promote T-cell activation.[83] HHV-7 is ubiquitous and is acquired in childhood. There is a single report of primary HHV-7 encephalitis in an immunocompetent adult, in whom HHV-7 DNA was found in the CSF,[84] and another report of meningitis and optic neuritis resulting from reactivation of HHV-7 in a stem cell transplant recipient.[85] HHV-7 infection in children has been linked with seizures and encephalitis through the detection of intrathecal synthesis of antibody against the virus.[86] Kaposi's Sarcoma-associated Herpesvirus (Human Herpesvirus 8) Kaposi's sarcoma-associated herpesvirus (KSHV or HHV-8) is the most recently discovered HHV. Using PCR-based subtractive analysis, Chang et al.[87] detected segments of the KSHV genome in tumor cells of Kaposi's sarcomas, the most common tumors seen in patients with AIDS. KSHV is also associated with primary effusion lymphoma and specific forms of multicentric Castleman disease, both of which are B-cell lymphoproliferative disorders. KSHV becomes latent in B cells. The viral genome is episomal, and its complete sequence (137 kilobase pairs) has been determined. Like EBV, KSHV encodes a latency-associated nuclear antigen, which tethers the virus episome to the host chromosome. This tethering not only ensures that virus DNA replication is synchronous with host chromosome replication, but also represses activation of virus genes associated with replication. Expression of a lytic HHV-8 gene that encodes a viral G-protein-coupled receptor can lead to the formation of Kaposi's sarcoma-like lesions in mice.[88] To date, no neurological disease has been associated with KSHV infection. Table 3. Management of herpes infection Disease Pharmacological Therapy Herpes Simplex Virus 1 Intravenous aciclovir (10 mg/kg body weight three times a day for 14–21 days) is the standard treatment for HSV-1 encephalitis in adults. Steroids are sometimes given as well, although data regarding the efficacy of this approach remain anecdotal. Cognitive impairment and seizures are significant neurological sequelae in treated patients. Herpes Simplex Virus 2 Intravenous aciclovir (10 mg/kg body weight three times a day for 14–21 days) is the standard treatment for HSV-1 encephalitis in adults. Steroids are sometimes given as well, although data regarding the efficacy of this approach remain anecdotal. Cognitive impairment and seizures are significant neurological sequelae in treated patients. Varicella Zoster Virus (Herpes Simplex Intravenous aciclovir (10 mg/kg body weight three times a day for 14–21 days) is the standard Virus 3) treatment for HSV-1 encephalitis in adults. Steroids are sometimes given as well, although data regarding the efficacy of this approach remain anecdotal. Cognitive impairment and seizures are significant neurological sequelae in treated patients. Epstein–Barr Virus (Herpes Simplex There is no known effective antiviral or other accepted treatment for EBV. Virus 4) Cytomegaloviru (Herpes Simplex Virus There have been no randomized controlled studies of antiviral therapy in adults with CMV infection 5) of the nervous system. An international panel that developed guidelines for treatment of CMV diseases in adults with AIDS receiving highly active antiretroviral therapy[73] recommended treatment with intravenous ganciclovir, intravenous foscarnet, or a combination of the two drugs. Patients who develop CMV polyradiculopathy while on ganciclovir or foscarnet therapy should receive combination therapy with both drugs. Patients not on treatment at the time of disease onset could be treated with either drug as monotherapy, and switched to combination therapy if they progressed clinically and/or had persisting CSF pleocytosis (a potential marker of drug failure). Reports of antiviral therapy for CMV-associated mononeuritis multiplex or painful symmetrical neuropathy are too limited to permit conclusions about efficacy of antiviral therapy. Herpes Simplex Virus 6 There have been no randomized controlled trials of antiviral therapies in either immunocompetent or immunocompromised patients. Ganciclovir and foscarnet have been used, but their efficacy is unknown. The outcome in immunocompetent patients does not appear to be substantially better than that in immunocompromised individuals. Real-time fluorescent probe CSF PCR has been used to follow the effects of antiviral therapy on HHV-6 DNA levels in the CSF of 11 hematopoietic stem cell recipients with HHV-6 infection (8 of whom had HHV-6 encephalitis).[82] All patients were treated with ganciclovir, foscarnet or both. Within 3 weeks of commencing antiviral therapy, median CSF viral load had decreased from 25,000 copies/ml to 100 copies/ml, although the difference was not statistically significant (P = 0.13). Four of the eight patients with HHV-6 encephalitis improved clinically, with viral load declining concurrently with antiviral therapy. Herpes Simplex Virus 7 NA Kaposi's Sarcoma-associated NA Herpesvirus (Herpes Simplex Virus 8) SUMMARY
- 12. SUMMARY Most HHVs can cause serious neurological disease of the PNS and CNS through primary infection or following virus reactivation from latently infected human ganglia or lymphoid tissue. The neurological complications include meningitis, encephalitis, myelitis, vasculopathy, acute and chronic radiculoneuritis, and various inflammatory diseases of the eye. Disease can be monophasic, recurrent or chronic. Recognition of the clinical patterns and imaging characteristics of disease produced by different herpesviruses is important, because infection by many of the herpesviruses can be treated successfully. Early diagnosis and proper treatment are essential to a favorable outcome. Virological confirmation of neurological disease relies on the detection of herpesvirus-specific DNA in bodily fluids or tissues, herpesvirus-specific IgM in blood, or herpesvirus-specific IgM or IgG antibody in cerebrospinal fluid. HSV-1, HSV-2, VZV and CMV are the most treatable herpesviruses. Table 4. The neurology of herpes viruses infection Virus Comment Herpes simplex virus 1 Herpes simplex virus 1 (HSV-1) encephalitis predominantly involves the orbital surface of the frontal lobes and medial surface of the temporal lobes, resulting in areas of increased T2 signal on MRI Herpes simplex virus 2 Herpes simplex virus 2 (HSV-2) is the primary cause of recurrent meningitis Varicella zoster virus After varicella, the varicella zoster virus (VZV) becomes latent in ganglia along the entire neuraxis; its reactivation (Human Herpesvirus 3) can lead to herpes zoster, vasculopathy, myelitis, necrotizing retinitis or zoster sine herpete Epstein–Barr virus The neurological complications of Epstein–Barr virus are diverse, and include meningitis, encephalitis, myelitis, (Human Herpesvirus 4) radiculoneuropathy, and even autonomic neuropathy cytomegalovirus (CMV) The most common neurological complication of cytomegalovirus (CMV) is poly-radiculoneuropathy in (Human Herpesvirus 5) immunocompromised individuals Human Herpesvirus 6 HHV-6 causes roseola, a common childhood exanthematous disease. Neurological complications can result from direct invasion of the CNS. HHV-6 DNA has been detected in the CSF of patients with febrile seizures[74] and in the brains of patients with fulminant hepatitis.[75] HHV-6 antigen was found in brain tissue from a fatal case of roseola. [76] HHV-6 causes encephalitis primarily after bone marrow or stem cell transplantation,[77] and might present as limbic encephalitis.[78] Human Herpesvirus 7 HHV-7 is closely related to HHV-6, and can induce reactivation of the latter in vitro. HHV-7 was first isolated from CD4+ T cells of a healthy 26-year-old under conditions that promote T-cell activation.[83] HHV-7 is ubiquitous and is acquired in childhood. There is a single report of primary HHV-7 encephalitis in an immunocompetent adult, in whom HHV-7 DNA was found in the CSF,[84 Human Herpesvirus 8 Kaposi's sarcoma-associated herpesvirus (KSHV or HHV-8) is the most recently discovered HHV. Using PCR-based subtractive analysis, Chang et al.[87] detected segments of the KSHV genome in tumor cells of Kaposi's sarcomas, the most common tumors seen in patients with AIDS. Addendum A new version of this PDF file (with a new case) is uploaded in my web site every week (every Saturday and remains available till Friday.) To download the current version follow the link "http://pdf.yassermetwally.com/case.pdf". You can also download the current version from my web site at "http://yassermetwally.com". To download the software version of the publication (crow.exe) follow the link: http://neurology.yassermetwally.com/crow.zip The case is also presented as a short case in PDF format, to download the short case follow the link: http://pdf.yassermetwally.com/short.pdf At the end of each year, all the publications are compiled on a single CD-ROM, please contact the author to know more details. Screen resolution is better set at 1024*768 pixel screen area for optimum display. For an archive of the previously reported cases go to www.yassermetwally.net, then under pages in the right panel, scroll down and click on the text entry "downloadable case records in PDF format" Also to view a list of the previously published case records follow the following link (http://wordpress.com/tag/case-record/) or click on it if it appears as a link in your PDF reader REFERENCES References 1.Lakeman FD and Whitley RJ (1995) Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis 171: 857-863 2.Weil AA et al. (2002) Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis 34: 1154-1157 3.Westmoreland BF (1987) The EEG in cerebral inflammatory processes. In Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, edn 2, 259-273 (Eds Niedermeyer E and Lopes da Silva FH) Baltimore-Munich: Urban and Schwarzenberg 4.Smith JB et al. (1975) A distinctive clinical EEG profile in herpes simplex encephalitis. Mayo Clin Proc 50: 469-474 5.Zimmerman RD et al. (1980) CT in the early diagnosis of herpes simplex encephalitis. Am J Radiol 134: 61-66 6.Schroth G et al. (1987) Early diagnosis of herpes simplex encephalitis by MRI. Neurology 37: 179-183
- 13. 7.Bastian FO et al. (1972) Herpesvirus hominis: isolation from human trigeminal ganglion. Science 178: 306-307 8.Baringer JR and Swoveland P (1973) Recovery of herpes simplex virus from human trigeminal ganglions. N Engl J Med 288: 648-650 9.Warren KG et al. (1978) Herpes simplex virus latency in patients with multiple sclerosis, lymphoma and normal humans. IARC Sci Publ 765- 768 10.Bustos DE and Atherton SS (2002) Detection of herpes simplex virus type 1 in human ciliary ganglia. Invest Ophthalmol Vis Sci 43: 2244-2249 11.Mahalingam R et al. (1992) Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol 31: 444-448 12.Fraser NW et al. (1981) Herpes simplex type 1 DNA in human brain tissue. Proc Natl Acad Sci USA 78: 6461-6465 13.Sawtell NM (1997) Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol 71: 5423-5431 14.Cohrs RJ et al. (2000) Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol 74: 11464-11471 15.Vrabec JT and Alford RL (2004) Quantitative analysis of herpes simplex virus in cranial nerve ganglia. J Neurovirol 10: 216-222 16.Rock DL and Fraser NW (1985) Latent herpes simplex virus type 1 DNA contains two copies of the virion DNA joint region. J Virol 55: 849- 852 17.Stevens JG et al. (1987) RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235: 1056- 1059 18.Kang W et al. (2003) Establishment and maintenance of HSV latent infection is mediated through correct splicing of the LAT primary transcript. Virology 312: 233-244 19.Izumi KM et al. (1989) Molecular and biological characterization of a type 1 herpes simplex virus (HSV-1) specifically deleted for expression of the latency-associated transcript (LAT). Microb Pathog 7: 121-134 20.Perng GC et al. (2000) Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 287: 1500-1503 21.Gupta A et al. (2006) Anti-apoptotic function of a microRNA encoded by the HSV-1 latency-associated transcript. Nature 442: 82-85 22.Bourne N et al. (2003) Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis 197: 542-549 23.Murakami S et al. (1996) Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med 124: 27-30 24.Furuta Y et al. (1992) Latent herpes simplex virus type 1 in human geniculate ganglia. Acta Neuropathol (Berl) 84: 39-44 25.Gonzales N et al. (2003) Recurrent dermatomal vesicular skin lesions: a clue to diagnosis of herpes simplex virus 2 meningitis. Arch Neurol 60: 868-869 26.Tedder DG et al. (1994) Herpes simplex virus infection as a cause of benign recurrent lymphocytic meningitis. Ann Intern Med 121: 334-338 27.Yamamoto LJ et al. (1991) Herpes simplex virus type 1 DNA in cerebrospinal fluid of a patient with Mollaret's meningitis. N Engl J Med 325: 1082-1085 28.Baringer JR (1974) Recovery of herpes simplex virus from human sacral ganglions. New Engl J Med 291: 828-830 29.Krohel GB et al. (1976) Herpes simplex neuropathy. Neurology 26: 596-597 30.Morris HH and Peters BH (1974) Recurrent sciatica associated with herpes simplex: case report. J Neurosurg 41: 97-99 31.Miller AE (1980) Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology 30: 582-587 32.Oxman MN et al. (2005) A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 352: 2271-2284 33.Gilden DH et al. (2005) VZV vasculopathy and postherpetic neuralgia: progress and perspective on antiviral therapy. Neurology 65: 21-25 34.Gilden DH et al. (1996) Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5-1995 revisited. Neurology 47: 1441-1446 35.Gilden DH et al. (2002) Two patients with unusual forms of varicella-zoster virus vasculopathy. N Eng J Med 347: 1500-1503 36.Gilden DH et al. (1998) The value of cerebrospinal fluid antiviral antibody in the diagnosis of neurologic disease produced by varicella zoster virus. J Neurol Sci 159: 140-144 37.Sato M et al. (1979) Herpes zoster of the maxillary branch of the trigeminus nerve: virological and serological studies. Int J Oral Surg 8: 149- 154 38.Vonsover A et al. (1987) Detection of varicella-zoster virus in lymphocytes of DNA hybridization. J Med Virol 21: 57-66
- 14. 39.Gilden DH et al. (1988) Persistence of varicella-zoster virus DNA in blood mononuclear cells of patients with varicella or zoster. Virus Genes 2: 299-305 40.Mahalingam R et al. (1995) Persistence of varicella-zoster virus DNA in elderly patients with postherpetic neuralgia. J Neurovirol 1: 130-133 41.Schott GC (1998) Triggering of delayed-onset postherpetic neuralgia. Lancet 351: 419-420 42.Vafai A et al. (1988) Expression of varicella-zoster virus blood mononuclear cells of patients with postherpetic neuralgia. Proc Natl Acad Sci 85: 2767-2770 43.Gilden DH et al. (1994) Zoster sine herpete, a clinical variant. Ann Neurol 34: 530-533 44.Terada K et al. (1998) Detection of varicella-zoster virus DNA in peripheral mononuclear cells from patients with Ramsay Hunt syndrome or zoster sine herpete. J Med Virol 56: 359-363 45.Yamada S et al. (2003) Ipsilateral truncal sensory deficit in a patient with ophthalmic zoster sine herpete. Neurology 60: 1049-1050 46.Hevner RF et al. (2003) An unusual cause of trigeminal distribution pain and tumour. Lancet Neurol 2: 567-571 47.Gilden DH et al. (2003) Chronic varicella zoster virus ganglionitis—a possible cause of postherpetic neuralgia. J Neurovirol 9: 404-407 48.Quan D et al. (2006) Improvement of postherpetic neuralgia after treatment with intravenous acyclovir followed by oral valacyclovir. Arch Neurol 63: 940-952 49.Devlin ME et al. (1992) Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J Infect Dis 165: 619-622 50.Gilden DH et al. (1983) Varicella-zoster virus DNA in human sensory ganglia. Nature 306: 478-480 51.Hyman RW et al. (1983) Varicella-zoster virus RNA in human trigeminal ganglia. Lancet 322: 814-816 52.Gilden DH et al. (1987) Detection of varicella-zoster virus nucleic acid in neurons of normal human thoracic ganglia. Ann Neurol 22: 377-380 53.Clarke P et al. (1995) Configuration of latent varicella-zoster virus DNA. J Virol 69: 8151-8154 54.Wood MJ et al. (1994) A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med 330: 896-900 55.Whitley RJ et al. (1996) Acyclovir with and without prednisone for the treatment of herpes zoster: a randomized, placebo-controlled trial. Ann Intern Med 125: 376-383 56.Bennett JL et al. (1996) Epstein-Barr virus-associated acute autonomic neuropathy. Ann Neurol 40: 453-455 57.Majid A et al. (2002) Epstein-Barr virus myeloradiculitis and encephalomyeloradiculitis. Brain 125: 159-165 58.Imai S et al. (1993) Epstein-Barr virus genomic sequences and specific antibodies in cerebrospinal fluid in children with neurologic complications of acute and reactivated EBV infections. J Med Virol 40: 278-284 59.Lehrnbecher T et al. (1996) Activated T lymphocytes in the cerebrospinal fluid of a patient with Epstein-Barr virus-associated meningoencephalitis. Pediatr Infect Dis J 15: 631-633 60.Baumgarten E et al. (1994) Life-threatening infectious mononucleosis: is it correlated with virus-induced T cell proliferation? Clin Infect Dis 19: 152-156 61.MacMahon EM et al. (1991) Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet 338: 969-973 62.Comoli P et al. (2005) Treatment of EBV-related post-renal transplant lymphoproliferative disease with a tailored regimen including EBV- specific T cells. Am J Transplant 5: 1415-1422 63.Cohen JI (2000) Epstein-Barr virus infection. N Engl J Med 343: 481-492 64.Rickinson A and Kieff E (2001) Epstein-Barr virus. In Virology, edn 4, 2573-2627 (Eds Knipe D et al.) Philadelphia: Lippincott Williams and Wilkins 65.Duchowny M et al. (1979) Cytomegalovirus infection of the adult nervous system. Ann Neurol 5: 458-461 66.Schneck SA (1965) Neuropathological features of human organ transplantation. I: probable cytomegalovirus infection. J Neuropathol Exp Neurol 24: 415-429 67.Eidelberg D et al. (1986) Progressive polyradiculopathy in acquired immune deficiency syndrome. Neurology 36: 912-916 68.Mocarski ES Jr and Courcelle CT (2001) Cytomegaloviruses and their replication. In Virology, edn 4, 2629-2673 (Eds Knipe D et al.) Philadelphia: Lippincott Williams and Wilkins 69.Rasmussen L et al. (2003) Inter- and intragenic variations complicate the molecular epidemiology of human cytomegalovirus. J Infect Dis 187: 809-819 70.Stinski MF et al. (1979) DNA of human cytomegalovirus: size heterogeneity and defectiveness resulting from serial undiluted passage. J Virol 31: 231-239
- 15. 71.Hummel M and Abecassis MM (2002) A model for reactivation of CMV from latency. J Clin Virol 25 (Suppl 2): S123-S136 72.Froberg MK (2004) CMV escapes! Ann Clin Lab Sci 34: 123-130 73.Whitley RJ et al. (1998) Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy. Arch Intern Med 158: 957-969 74.Dewhurst S (2004) Human herpesvirus type 6 and human herpesvirus type 7 infections of the central nervous system. Herpes 11 (Suppl 2): S105A-S111A 75.Asano Y et al. (1990) Fatal fulminant hepatitis in an infant with human herpesvirus-6 infection. Lancet 335: 862-863 76.Portolani M et al. (2005) Post-mortem diagnosis of encephalitis in a 75-year-old man associated with human herpesvirus-6 variant A. J Med Virol 77: 244-248 77.Zerr DM et al. (2001) Human herpesvirus 6 reactivation and encephalitis in allogeneic bone marrow transplant recipients. Clin Infect Dis 33: 763-771 78.Wainwright MS et al. (2001) Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol 50: 612-619 79.Kondo K et al. (1991) Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol 72: 1401-1408 80.Kong H et al. (2003) Human herpesvirus type 6 indirectly enhances oligodendrocyte cell death. J Neurovirol 9: 539-550 81.Dietrich J et al. (2004) Infection with an endemic human herpesvirus disrupts critical glial precursor cell properties. J Neurosci 24: 4875-4883 82.Zerr DM et al. (2002) Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin Infect Dis 34: 309-317 83.Frenkel N et al. (1990) Isolation of a new herpesvirus from human CD4+ T cells. Proc Natl Acad Sci USA 87: 748-752 84.Ward KN et al. (2002) Neuroinvasion during delayed primary HHV-7 infection in an immunocompetent adult with encephalitis and flaccid paralysis. J Med Virol 67: 538-541 85.Yoshikawa T et al. (2003) Human herpesvirus 7-associated meningitis and optic neuritis in a patient after allogeneic stem cell transplantation. J Med Virol 70: 440-443 86.Yoshikawa T et al. (2000) Invasion by human herpesvirus 6 and human herpesvirus 7 of the central nervous system in patients with neurological signs and symptoms. Arch Dis Child 83: 170-171 87.Chang Y et al. (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266: 1865-1869 88.Montaner S et al. (2006) The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor as a therapeutic target for the treatment of Kaposi's sarcoma. Cancer Res 66: 168-174 89. Metwally, MYM: Textbook of neuroimaging, A CD-ROM publication, (Metwally, MYM editor) WEB-CD agency for electronic publication, version 11.1a. January 2010
