Chapter #3 Lectures Part Ii
•Télécharger en tant que PPT, PDF•
0 j'aime•529 vues
Signaler
Partager
Signaler
Partager
Recommandé
Biocatalysts are substance which alters to promote the reaction and a substance especially an enzyme, that initiates or modified the rate of chemical reaction.Role of biocatalysts in green synthesis , green chemistry

Role of biocatalysts in green synthesis , green chemistryMinal Saini , student of Chaudhary bansilal University , Bhiwani, Haryana
Recommandé
Biocatalysts are substance which alters to promote the reaction and a substance especially an enzyme, that initiates or modified the rate of chemical reaction.Role of biocatalysts in green synthesis , green chemistry

Role of biocatalysts in green synthesis , green chemistryMinal Saini , student of Chaudhary bansilal University , Bhiwani, Haryana
IJRET : International Journal of Research in Engineering and Technology is an international peer reviewed, online journal published by eSAT Publishing House for the enhancement of research in various disciplines of Engineering and Technology. The aim and scope of the journal is to provide an academic medium and an important reference for the advancement and dissemination of research results that support high-level learning, teaching and research in the fields of Engineering and Technology. We bring together Scientists, Academician, Field Engineers, Scholars and Students of related fields of Engineering and TechnologySynthesis, characterisation and antibacterial activity of copolymer (n vinylp...

Synthesis, characterisation and antibacterial activity of copolymer (n vinylp...eSAT Publishing House
Contenu connexe
Tendances
IJRET : International Journal of Research in Engineering and Technology is an international peer reviewed, online journal published by eSAT Publishing House for the enhancement of research in various disciplines of Engineering and Technology. The aim and scope of the journal is to provide an academic medium and an important reference for the advancement and dissemination of research results that support high-level learning, teaching and research in the fields of Engineering and Technology. We bring together Scientists, Academician, Field Engineers, Scholars and Students of related fields of Engineering and TechnologySynthesis, characterisation and antibacterial activity of copolymer (n vinylp...

Synthesis, characterisation and antibacterial activity of copolymer (n vinylp...eSAT Publishing House
Tendances (6)
Synthesis, characterisation and antibacterial activity of copolymer (n vinylp...

Synthesis, characterisation and antibacterial activity of copolymer (n vinylp...
Crosslinked Microgels as Platform for Hydrolytic Catalysts Article pubs.acs.o...

Crosslinked Microgels as Platform for Hydrolytic Catalysts Article pubs.acs.o...
SYNTHESIS OF BIOACTIVE HETEROCYCLES BY USING SONOCHEMISTRY

SYNTHESIS OF BIOACTIVE HETEROCYCLES BY USING SONOCHEMISTRY
En vedette
En vedette (18)
2011 topic 01 lecture 3 - limiting reactant and percent yield

2011 topic 01 lecture 3 - limiting reactant and percent yield
Rate of reaction =measure rate and intro and collision theory

Rate of reaction =measure rate and intro and collision theory
K to 12 Mathematics Curriculum Guide for Grades 1 to 10

K to 12 Mathematics Curriculum Guide for Grades 1 to 10
Similaire à Chapter #3 Lectures Part Ii
Similaire à Chapter #3 Lectures Part Ii (20)
Dernier
Dernier (20)
A Business-Centric Approach to Design System Strategy

A Business-Centric Approach to Design System Strategy
Where to Learn More About FDO _ Richard at FIDO Alliance.pdf

Where to Learn More About FDO _ Richard at FIDO Alliance.pdf
Choosing the Right FDO Deployment Model for Your Application _ Geoffrey at In...

Choosing the Right FDO Deployment Model for Your Application _ Geoffrey at In...
SOQL 201 for Admins & Developers: Slice & Dice Your Org’s Data With Aggregate...

SOQL 201 for Admins & Developers: Slice & Dice Your Org’s Data With Aggregate...
UiPath Test Automation using UiPath Test Suite series, part 1

UiPath Test Automation using UiPath Test Suite series, part 1
Integrating Telephony Systems with Salesforce: Insights and Considerations, B...

Integrating Telephony Systems with Salesforce: Insights and Considerations, B...
10 Differences between Sales Cloud and CPQ, Blanka Doktorová

10 Differences between Sales Cloud and CPQ, Blanka Doktorová
Linux Foundation Edge _ Overview of FDO Software Components _ Randy at Intel.pdf

Linux Foundation Edge _ Overview of FDO Software Components _ Randy at Intel.pdf
Agentic RAG What it is its types applications and implementation.pdf

Agentic RAG What it is its types applications and implementation.pdf
Measures in SQL (a talk at SF Distributed Systems meetup, 2024-05-22)

Measures in SQL (a talk at SF Distributed Systems meetup, 2024-05-22)
Extensible Python: Robustness through Addition - PyCon 2024

Extensible Python: Robustness through Addition - PyCon 2024
Chapter #3 Lectures Part Ii
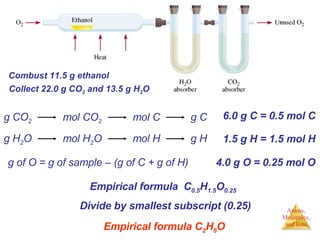
- 1. g of O = g of sample – (g of C + g of H) Combust 11.5 g ethanol Collect 22.0 g CO 2 and 13.5 g H 2 O 6.0 g C = 0.5 mol C 1.5 g H = 1.5 mol H 4.0 g O = 0.25 mol O Empirical formula C 0.5 H 1.5 O 0.25 Divide by smallest subscript (0.25) Empirical formula C 2 H 6 O g CO 2 mol CO 2 mol C g C g H 2 O mol H 2 O mol H g H
- 2. A process in which one or more substances is changed into one or more new substances is a chemical reaction A chemical equation uses chemical symbols to show what happens during a chemical reaction
- 3. How to “Read” Chemical Equations 2 Mg + O 2 2 MgO
- 10. Methanol burns in air according to the equation If 209 g of methanol are used up in the combustion, what mass of water is produced? 2CH 3 OH + 3O 2 2CO 2 + 4H 2 O
- 14. In one process, 124 g of Al are reacted with 601 g of Fe 2 O 3 Calculate the mass of Al 2 O 3 formed. 2Al + Fe 2 O 3 Al 2 O 3 + 2Fe
- 15. Use limiting reagent (Al) to calculate amount of product that can be formed.
- 16. Theoretical Yield is the amount of product that would result if all the limiting reagent reacted. Actual Yield is the amount of product actually obtained from a reaction.