Signaler
Partager
Télécharger pour lire hors ligne
Recommandé
Recommandé
Contenu connexe
Similaire à amina presentation.pdff
Similaire à amina presentation.pdff (20)
WASTEWATER TREATMENT TECHNOLOGIES FOR THE REMOVAL OF NITROGEN & PHOSPHORUS 

WASTEWATER TREATMENT TECHNOLOGIES FOR THE REMOVAL OF NITROGEN & PHOSPHORUS
kfcl.pptx for training and study and understanding

kfcl.pptx for training and study and understanding
Fertiliser from air and water with low carbon electricity

Fertiliser from air and water with low carbon electricity
Plus de kashafAzam1
Plus de kashafAzam1 (6)
Dernier
Dernier (20)
Nightside clouds and disequilibrium chemistry on the hot Jupiter WASP-43b

Nightside clouds and disequilibrium chemistry on the hot Jupiter WASP-43b
TEST BANK For Radiologic Science for Technologists, 12th Edition by Stewart C...

TEST BANK For Radiologic Science for Technologists, 12th Edition by Stewart C...
Recombinant DNA technology (Immunological screening)

Recombinant DNA technology (Immunological screening)
Asymmetry in the atmosphere of the ultra-hot Jupiter WASP-76 b

Asymmetry in the atmosphere of the ultra-hot Jupiter WASP-76 b
High Class Escorts in Hyderabad ₹7.5k Pick Up & Drop With Cash Payment 969456...

High Class Escorts in Hyderabad ₹7.5k Pick Up & Drop With Cash Payment 969456...
Labelling Requirements and Label Claims for Dietary Supplements and Recommend...

Labelling Requirements and Label Claims for Dietary Supplements and Recommend...
Stunning ➥8448380779▻ Call Girls In Panchshil Enclave Delhi NCR

Stunning ➥8448380779▻ Call Girls In Panchshil Enclave Delhi NCR
Hubble Asteroid Hunter III. Physical properties of newly found asteroids

Hubble Asteroid Hunter III. Physical properties of newly found asteroids
Forensic Biology & Its biological significance.pdf

Forensic Biology & Its biological significance.pdf
Disentangling the origin of chemical differences using GHOST

Disentangling the origin of chemical differences using GHOST
Pests of mustard_Identification_Management_Dr.UPR.pdf

Pests of mustard_Identification_Management_Dr.UPR.pdf
Chemical Tests; flame test, positive and negative ions test Edexcel Internati...

Chemical Tests; flame test, positive and negative ions test Edexcel Internati...
9654467111 Call Girls In Raj Nagar Delhi Short 1500 Night 6000

9654467111 Call Girls In Raj Nagar Delhi Short 1500 Night 6000
Formation of low mass protostars and their circumstellar disks

Formation of low mass protostars and their circumstellar disks
Creating and Analyzing Definitive Screening Designs

Creating and Analyzing Definitive Screening Designs
❤Jammu Kashmir Call Girls 8617697112 Personal Whatsapp Number 💦✅.

❤Jammu Kashmir Call Girls 8617697112 Personal Whatsapp Number 💦✅.
amina presentation.pdff
- 2. Group Members: • Amina Younas BS(ADP)E-Chem-021 • Sabahat Fatima BS(ADP)E-Chem-022 • Palwasha Sajid BS(ADP)E-Chem-023
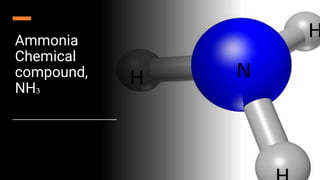
- 3. Introduction Ammonia is a compound of nitrogen and hydrogen with the formula NH 3 . Ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms
- 4. Raw Materials Hydrogen in the form of H₂ Nitrogen in the form of N₂ Hydrogen is obtained by reacting natural gas (mostly methane) with steam, or from cracking oil fractions. Nitrogen is obtained from the air.
- 5. On industrial scale Production of Ammonia
- 6. HABER’S PROCESS • The Haber process (sometimes referred to as the Haber-Bosch process) is an industrial procedure for obtaining ammonia from nitrogen an d hydrogen in the gaseous state. The Haber method is done under high temperature and pressure conditions and can be described by the following reverse reaction: • N2(g)+3 H2(g)⇌2 NH3(g)
- 7. Process : 1. First, we take nitrogen gas from the air and combine it with hydrogen atom obtained from natural gas in the ratio 1:3 by volume. 2. The gases are passed through four beds of catalyst, with cooling takes place in each pass. This is done to maintain equilibrium constant. 3. While different levels of conversion occur in each pass where unreacted gases are recycled. 4. Normally an iron catalyst is used in the process, and the whole procedure is conducted by maintaining a temperature of around 400 – 450oC and a pressure of 150 – 200 atm. 5. The process also involves steps like shift conversion, carbon dioxide removal, steam reforming, and methanation. 6. In the final stage of the process, the ammonia gas is cooled down to form a liquid solution which is then collected and stored in storage containers.
- 9. Conditions for Haber’s process • Minimizing cost of production by ensuring • (a) The capital cost of the plant is not too high, • (b) The starting materials are cheap; • Maximizing yield of products by • (a) Shifting the equilibrium position in the desired direction • (b) Increasing the value of the equilibrium constant,K,for the process concerned;
- 10. Reaction Rate and Equilibrium • The Haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. The chemical reaction is given below. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. • N2(g) + 3H2(g) → 2NH3(g)
- 11. Electrochemical Synthesis of Ammonia: • The dominant process for ammonia synthesis is the Haber-Bosch (H-B) process which involves the production of H2 from the steam- reforming of natural gas, or coals, which concurrently produces enormous amounts of CO2. This is followed by extensive purification of this H2, before its reaction with N2 at 400– 500 °C and at elevated pressures (about 150 bar) over a Fe-based catalyst [1,2]. Ever since its discovery, the H-B process has been gradually improved upon. The improvements consisted primarily in searching for more active catalysts which would allow operation at lower pressures and temperatures.
- 12. Biological Problems / Difficulties: • Temperature: Toxicity of ammonia (as total ammonia) increases as temperature increases (U.S. EPA 1999). • pH: Ammonia concentration and toxicity increases as pH increases, although less ammonia is required to produce toxic effects at lower pH (IPCS 1986, Wurts 2003). • Dissolved oxygen: Oxygen is consumed as ammonia is oxidized (nitrification), and low oxygen levels increase ammonia levels by inhibiting nitrification. • Season: Total ammonia-nitrogen concentration in surface waters tends to be lower during summer than during winter. This is due to uptake by plants and decreased ammonia solubility at higher water temperatures (IPCS 1986). • Ionic strength: Tolerance to ammonia can increase with an increase in ionic strength or salinity (Sampaio et al. 2002). • Sediments: Fine sediments tend to generate ammonia due to low oxygen levels and high organic matter.
- 13. Backup Option : GREEN AMMONIA • What is Green Ammonia? • Green ammonia refers to ammonia, which has been produced through a process that is 100% renewable and carbon-free. One way of making Green Ammonia is by using the hydrogen from water electrolysis and nitrogen separated from air. These two elements are then fed into the Haber process. In the process, nitrogen and hydrogen react together in high pressure and temperature to produce Ammonia. • Currently, ammonia making is not a green process. It is now made from methane. It is called Steam Methane Reforming (SMR) process. Around 90% of carbon dioxide is produced from SMR process.
- 14. Flow Sheet Diagram of Production of Green Ammonia
- 15. Uses of Ammonia : As a FERTILIZER • •About 90 percent of ammonia produced is used in fertilizer to help sustain food production for billions of people around the world. Specially ammonium sulphate has many profound properties that plays a vital role in plants growth and also in controlling soil salinity.
- 16. Household uses • Ammonia is one of the main ingredients in a lot of household cleaning products. It is used as a cleaning agent and can be used to remove stains or clean mirrors, tubs, sinks, windows and more. Some other uses include antimicrobial agent or an antiseptic, and ammonia is also used as a fuel.
- 18. Risk factors while working with ammonia • At room temperature,ammonia is a colorless,highly irritating gas with a pungent,suffocating odor. • In pure form,it is known as anhydrous ammonia and is hygroscopic (readily absorbs moisture). • Ammonia has alkaline properties and is corrosive. • Ammonia gas dissolves easily in water to form ammonium hydroxide,a caustic solution and weak base.
- 19. Thankyou!