BM_FI.ppt
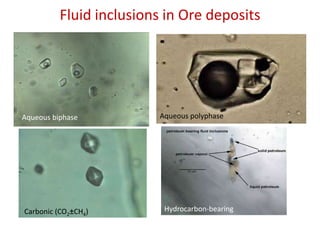
- 1. Fluid inclusions in Ore deposits Aqueous biphase Aqueous polyphase Carbonic (CO2±CH4) Hydrocarbon-bearing
- 2. Micro-geological systems, tiny cavities filled with representative of ore forming fluid regimes-ANCIENT HYDROTHERMAL SYSTEMS or ore deposits (modern hydrothermal systems). Furnish data (composition, P, T, salinity, density) Types : based on chronology Primary: trapped during crystal growth Secondary (trans-granular): formed after the crystal growth, along healed cracks Pseudo-secondary (intra-granular): occur along healed cracks and fractures that terminate at the grain boundaries cracks formed during xl growth. PRACTICALLY FURNISH THE SAME DATA as primary inclusions ANY INCLUSION WHICH IS TRAPPED ALONG THE PRIMARY GROWTH ZONE IN A MINERAL GRAIN IS PRIMARY. ISOLATED INCLUSIONS FORMING A RANDOM 3-D NETWORK IN UNDEFORMED HOST MINERAL GRAIN IS ALSO CONSIDERED AS PRIMARY
- 3. Assumptions Homogeneous entrapment - proof: inclusions showing apparently same phase ratios No change in cavity volume after entrapment Inclusions behaved as thermodynamically closed systems L V Aqueous bi- phase inclusion Aqueous- carbonic (gaseous) inclusion LH2O LCO2 VCO2
- 4. Any process, which interferes with the growth of a perfect crystal, may cause trapping of primary inclusions A period of rapid crystal growth forming a porous dendritic layer succeeded by a slower growth controlled cystallizationn, thus covering solid impervious layers and trapping many inclusions If the material (nutrients) is supplied to the growing crystal faces by a mass flow of fluid, large inclusion may be trapped as a result of temporary starvation of centre of faces relative to faster growing edges (having easier access to the fluid) the excess fluid is trapped If some crystal-growing blocks grow faster than others the surface becomes rough with many angular reentrants, which are filled in by fluids during later growth periods yielding negative crystal cavities Mechanisms of trapping
- 5. Phase changes since trapping SHRINKAGE: leads to formation of the vapour bubble due to differential shrinkage during cooling. Reversed in the lab by heating and temperature (Th) can be determined. Types: L + V L, L + V V, CRITICAL HOMOGENIZATION: by fading of the L- V meniscus DAUGHTER MINERALS: Fluid could become saturated wrt some dissolved electrolytes (salts) and they nucleate as daughter minerals (NaCl, KCl, CaSO4 etc) dissolve (Ts,NaCl etc.) during heating – contrary to captive phases METASTABILITY: Inclusions are very small system even from the atomic viewpoint. An inclusion of 10 size containing 30 solution of NaCl may have only 10111012 molecules of NaCl. Similarly, an inclusion containing 10 ppm PbS of the same size would contain around 25x106 molecules of PbS results in metastability i.e., failure to nucleate new but stable phases. Examples: failure to nucleate ice, NaCl.2H2O, CO25.75 H2O etc)
- 6. Most commonly used non-destructive analytical technique involving careful observation of phase changes (e.g., ice melting, homogenization, solid dissolution etc) as a function of temperature (195C to 700C) in individual inclusions, carried out in microscopic heating-freezing stages The data obtained can be used with the help of experimental data in pertinent systems to constrain the chemical (salinity, gross chemistry) and physical (density, P, T) parameters of the fluid Semi-quantitative constraints for comparing complex multi- component natural fluids to simplified experimental systems Microthermometry
- 7. H2O-NaCl system For inclusion A (Tm= 10C), has a salinity of 13.9 wt. % NaCl Wt. % NaCl = (1.78 Tm) [0.042 (Tm)2] [0.00057 (Tm)3] For inclusions containing > 26.3 wt % NaCl must have a halite daughter xl stable at room temp. Bulk salinity of such incl. is calculated from the halite dissolution temperature (Ts,NaCl) Wt % NaCl = 26.242 + (0.4928 Ts) + [1.42 (Ts)2] [0.223 (Ts)3] + [0.04129 (Ts)4] + [0.006295 (Ts)5] [0.001967 (Ts)6]+ [0.00011112 (Ts)7] where Ts = Ts,NaCl/ 100
- 8. Freezing: inclusion composition All phase changes occurring below room temperature Freezing is complimentary to heating and each inclusion should be frozen and heated to complete the microthermometric runs Data obtained on freezing primarily refer to gross fluid composition and density. For multi-component fluids the normal sequence during freezing is L+V S+L+V S+V. The melting temperature is a direct function of composition–thus fluid composition can be determined provided appropriate exptl. data (eutectic) are available Inherent problem– Reluctance to freeze due to metastability, the extent of which is inversely proportional to inclusion size
- 9. Eutectic first melting temperatures for selected H2O- salt systems SYSTEM Eutectic temp. (C) ------------------------------------------------------------------------------------ H2O- NaCl- CaCl2- MgCl2 57 H2O- NaCl- CaCl2 52 H2O- CaCl2 49.5 H2O- FeCl2 35 H2O- MgCl2 33.6 H2O- NaCl- KCl 23 H2O- NaCl 21.2 H2O- KCl 10.6
- 10. Sequence of photographs showing major phase changes observed in biphase (L+V) in fluorite. The fluid composition is in the H2O-NaCl-CaCl2 system
- 11. Homogenization temperature (Th) and isochore P- T diagram for pure H2O showing the heating paths taken by two inclusions A and B both homogenizing at the same temp (Th), but having different bulk density. A: L+V L (d > dc) and B: L+V V (d< dc). TtTh = pressure correction. Th is the minimum temperature
- 12. Influence of salinity on the boiling curve and slope of the isochores in the H2O- NaCl system With increasing density pressure correction increases
- 13. Since boiling in hydrothermal systems is essentially a near-isothermal decompression (or near-isobaric cooling) process, inclusions showing both liquid- as well as vapor- state homogenization is indicative of entrapment from a boiling fluid no pressure correction Fluid boiling
- 14. The daughter minerals (halite, sylvite) dissolve at different rates while heating the inclusion (KCl faster). For a saturated NaCl–H2O system, the final solution temperature of halite (TS,NaCl) is directly proportional to the wt % NaCl in the solution Dissolution of daughter minerals
- 15. NON-AQUEOUS CO2 (CH4) SYSTEMS VCO2 Sequence of phase changes in a pure CO2 inclusion during freezing-heating run finally homogenizing as liquid CO2 (LCO2 + VCO2 LCO2) CO2 ice 20C 100C 56.6C 27C 29C
- 16. PURE CO2 SYSTEM Density and homogenization temperature (Th,CO2) relationship in carbonic inclusions Density is calculated from the observed CO2 homogenization temperature, depending on the nature of homogenization
- 17. CO2- CH4 SYSTEM Phase relations in the CO2-CH4 system at sub-ambient temperature. The triple point of CO2 is lowered with increasing CH4 (Tm,CO2 in equilibria with LCO2 and VCO2 is depressed)
- 18. Analyzing gaseous (± H2O) by Raman Spectroscopy Presence of H2O and contents of CO2 and CH4 (XCO2, XCH4) can be determined, which can be used to calculate density and construct isochores in the CO2CH4 system
- 19. Coeval and cogenetic pure aqueous and carbonic fluid inclusions P-T estimation by Isochore intersection: FI thermobarometry
- 20. Fluid Evolution Isothermal mixing: Metamorphic/ magmatic fluid with heated meteoric water Mixing with cooler- less saline fluid: Magmatic with metamorphic/ evolved connate water