Thermal Energy
•Télécharger en tant que PPT, PDF•
5 j'aime•1,640 vues
Introduction to thermal energy
Signaler
Partager
Signaler
Partager
Recommandé
Recommandé
Contenu connexe
Tendances
Tendances (20)
HEATING AND COOLING (TEMPERATURE AND THERMAL ENERGY) LESSON ONE

HEATING AND COOLING (TEMPERATURE AND THERMAL ENERGY) LESSON ONE
Similaire à Thermal Energy
Similaire à Thermal Energy (20)
heat capacity and specific heat capacitylecture.pptx

heat capacity and specific heat capacitylecture.pptx
Dernier
God is a creative God Gen 1:1. All that He created was “good”, could also be translated “beautiful”. God created man in His own image Gen 1:27. Maths helps us discover the beauty that God has created in His world and, in turn, create beautiful designs to serve and enrich the lives of others.
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...christianmathematics
Making communications land - Are they received and understood as intended? webinar
Thursday 2 May 2024
A joint webinar created by the APM Enabling Change and APM People Interest Networks, this is the third of our three part series on Making Communications Land.
presented by
Ian Cribbes, Director, IMC&T Ltd
@cribbesheet
The link to the write up page and resources of this webinar:
https://www.apm.org.uk/news/making-communications-land-are-they-received-and-understood-as-intended-webinar/
Content description:
How do we ensure that what we have communicated was received and understood as we intended and how do we course correct if it has not.Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...Association for Project Management
Dernier (20)
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...
Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...

Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...
Unit-IV; Professional Sales Representative (PSR).pptx

Unit-IV; Professional Sales Representative (PSR).pptx
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...
Thermal Energy
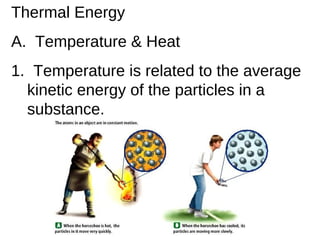
- 1. Thermal Energy A. Temperature & Heat 1. Temperature is related to the average kinetic energy of the particles in a substance.
- 2. 2. SI unit for temp. is the Kelvin a. K = C + 273 (10C = 283K) b. C = K – 273 (10K = -263C) 3. Thermal Energy – the total of all the kinetic and potential energy of all the particles in a substance.
- 3. 4. Thermal energy relationships a. As temperature increases, so does thermal energy (because the kinetic energy of the particles increased). b. Even if the temperature doesn’t change, the thermal energy in a more massive substance is higher (because it is a total measure of energy).
- 4. 5. Heat a. The flow of thermal energy from one object to another. b. Heat always flows from warmer to cooler objects. Ice gets warmer while hand gets cooler Cup gets cooler while hand gets warmer
- 5. 6. Specific Heat a. Some things heat up or cool down faster than others. Land heats up and cools down faster than water
- 6. b. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). 1) C water = 4184 J / kg C 2) C sand = 664 J / kg C This is why land heats up quickly during the day and cools quickly at night and why water takes longer.
- 7. Why does water have such a high specific heat? Water molecules form strong bonds with each other; therefore it takes more heat energy to break them. Metals have weak bonds and do not need as much energy to break them. water metal
- 8. How to calculate changes in thermal energy Q = m x T x C p Q = change in thermal energy m = mass of substance T = change in temperature (T f – T i ) C p = specific heat of substance
- 9. c. A calorimeter is used to help measure the specific heat of a substance. First, mass and temperature of water are measured Then heated sample is put inside and heat flows into water T is measured for water to help get its heat gain This gives the heat lost by the substance Knowing its Q value, its mass, and its T, its C p can be calculated