Signaler
Partager
Télécharger pour lire hors ligne
Recommandé
Recommandé
Contenu connexe
Similaire à 9.2 periodic trends-qp
Similaire à 9.2 periodic trends-qp (20)
10/13 Review: What is electronegativity and ionization energy?

10/13 Review: What is electronegativity and ionization energy?
F.sc.Part.2.Chemistry.(Chapter Wise Tests& Their Solution) - Malik Xufyan

F.sc.Part.2.Chemistry.(Chapter Wise Tests& Their Solution) - Malik Xufyan
F.sc.Part.2.Chemistry.(Chapter Wise Tests& Their Solution) - Malik Xufyan

F.sc.Part.2.Chemistry.(Chapter Wise Tests& Their Solution) - Malik Xufyan
F.sc. Chemistry Part 2. (inorganic portion tests & solved - Malik Xufyan

F.sc. Chemistry Part 2. (inorganic portion tests & solved - Malik Xufyan
revision on chapter periodic table, chemical bonding and electrolysis with an...

revision on chapter periodic table, chemical bonding and electrolysis with an...
Chapter 6- the periodic table 1-metal 2- Halogen 3- I.docx

Chapter 6- the periodic table 1-metal 2- Halogen 3- I.docx
Plus de yasminexxy1
Plus de yasminexxy1 (20)
12.1 acids-bases-and-salts-cie-igcse-chemistry-practical-qp

12.1 acids-bases-and-salts-cie-igcse-chemistry-practical-qp
13.1 identification-of-ions-and-gases-cie-igcse-chemistry-practical-qp

13.1 identification-of-ions-and-gases-cie-igcse-chemistry-practical-qp
12.1 acids _bases_and_salts_qp_-_igcse_cie_chemistry_-_ext_theory_paper

12.1 acids _bases_and_salts_qp_-_igcse_cie_chemistry_-_ext_theory_paper
12.2 acids _bases_and_salts_qp_-_igcse_cie_chemistry_-_ext_theory_paper

12.2 acids _bases_and_salts_qp_-_igcse_cie_chemistry_-_ext_theory_paper
14.1 coordination and-response-qp_igcse-cie-biology_

14.1 coordination and-response-qp_igcse-cie-biology_
14.2 coordination and-response-qp_igcse-cie-biology_

14.2 coordination and-response-qp_igcse-cie-biology_
14.1 coordination and-response-_igcse-cie-biology_-ext-theory-qp

14.1 coordination and-response-_igcse-cie-biology_-ext-theory-qp
14.2 coordination and-response-_igcse-cie-biology_-ext-theory-qp

14.2 coordination and-response-_igcse-cie-biology_-ext-theory-qp
Dernier
https://app.box.com/s/7hlvjxjalkrik7fb082xx3jk7xd7liz3TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...Nguyen Thanh Tu Collection
Dernier (20)
ICT role in 21st century education and it's challenges.

ICT role in 21st century education and it's challenges.
TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...
Beyond_Borders_Understanding_Anime_and_Manga_Fandom_A_Comprehensive_Audience_...

Beyond_Borders_Understanding_Anime_and_Manga_Fandom_A_Comprehensive_Audience_...
HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx

HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx
Micro-Scholarship, What it is, How can it help me.pdf

Micro-Scholarship, What it is, How can it help me.pdf
Interdisciplinary_Insights_Data_Collection_Methods.pptx

Interdisciplinary_Insights_Data_Collection_Methods.pptx
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
Sensory_Experience_and_Emotional_Resonance_in_Gabriel_Okaras_The_Piano_and_Th...

Sensory_Experience_and_Emotional_Resonance_in_Gabriel_Okaras_The_Piano_and_Th...
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
HMCS Vancouver Pre-Deployment Brief - May 2024 (Web Version).pptx

HMCS Vancouver Pre-Deployment Brief - May 2024 (Web Version).pptx
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
9.2 periodic trends-qp
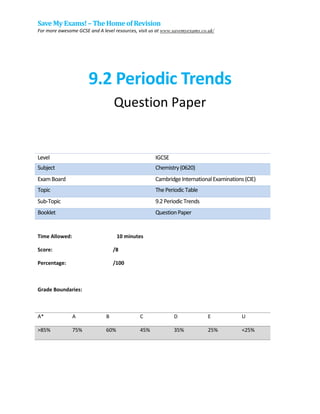
- 1. Save My Exams! – The Homeof Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk/ 9.2 Periodic Trends Question Paper Level IGCSE Subject Chemistry(0620) ExamBoard CambridgeInternationalExaminations(CIE) Topic ThePeriodicTable Sub-Topic 9.2PeriodicTrends Booklet QuestionPaper Time Allowed: 10 minutes Score: /8 Percentage: /100 Grade Boundaries: A* A B C D E U >85% 75% 60% 45% 35% 25% <25%
- 2. 1 Where in the Periodic Table is the metallic character of the elements greatest? 2 Argon, Ar, has a higher relative atomic mass than potassium, K, but appears before it in the Periodic Table. K Ar Why is argon listed before potassium in the Periodic Table? A Argon has fewer neutrons than potassium. B Argon has fewer protons than potassium. C Argon has more neutrons than potassium. D Argon has more protons than potassium. Save My Exams! – The Homeof Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk/
- 3. 3 J and K are two elements from the same period in the Periodic Table. The table gives some properties of J and K. Which statement about J and K is correct? A J forms an acidic oxide. B J is found to the left of K in the Periodic Table. C K forms positive ions when it reacts. D K is more metallic than J. 4 In the outline of the Periodic Table below, some elements are shown as numbers. 65 1 7 2 43 Which two numbers are metals in the same period? A 1 and 2 B 1 and 7 C 3 and 5 D 5 and 6 Save My Exams! – The Homeof Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk/
- 4. 5 Calcium, on the left of Period 4 of the Periodic Table, is more metallic than bromine on the right of this period. Why is this? Calcium has A fewer electrons. B fewer protons. C fewer full shells of electrons. D fewer outer shell electrons. 6 The diagram shows one period of the Periodic Table. i BLiL B C N O F Ne Which two elements form acidic oxides? A carbon and lithium B carbon and neon C carbon and nitrogen D nitrogen and neon 7 Which property of elements increases across a period of the Periodic Table? A metallic character B number of electron shells C number of outer shell electrons D tendency to form positive ions Save My Exams! – The Homeof Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk/
- 5. 8 W, X, Y and Z are elements in the same period in the Periodic Table. W and Y are metals. X and Z are non-metals. Which shows the correct order of these elements across the period? A W X Y Z B X Z W Y C Y W X Z D W Y X Z Save My Exams! – The Homeof Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk/