Lab Report on copper cycle
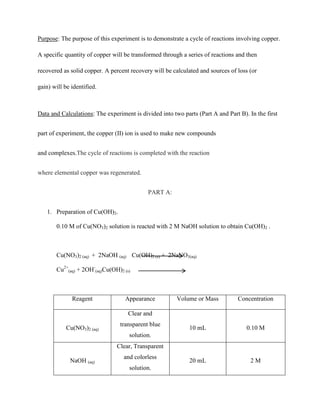
- 1. Purpose: The purpose of this experiment is to demonstrate a cycle of reactions involving copper. A specific quantity of copper will be transformed through a series of reactions and then recovered as solid copper. A percent recovery will be calculated and sources of loss (or gain) will be identified. Data and Calculations: The experiment is divided into two parts (Part A and Part B). In the first part of experiment, the copper (II) ion is used to make new compounds and complexes.The cycle of reactions is completed with the reaction where elemental copper was regenerated. PART A: 1. Preparation of Cu(OH)2. 0.10 M of Cu(NO3)2 solution is reacted with 2 M NaOH solution to obtain Cu(OH)2 . Cu(NO3)2 (aq) + 2NaOH (aq) Cu(OH)2 (s) + 2NaNO3(aq) Cu2+(aq) + 2OH-(aq)Cu(OH)2 (s) Reagent Appearance Volume or Mass Concentration 10 mL 0.10 M 20 mL 2M Clear and Cu(NO3)2 (aq) transparent blue solution. Clear, Transparent NaOH (aq) and colorless solution.
- 2. Observations: When we add the NaOH solution into the 10 mL Cu(NO3)2 the clear and transparent light blue color solution turns into dark blue mixture. The color of mixture becomes light after adding the distilled water. 2. Preparation of CuO: This step is completed using Bunsen burner. The whole mixture of Cu(OH)2 is placed over wire gauge for heating. Cu(OH)2(s, blue) + heat CuO (s, black) + H2O (l) The black precipitates of CuO settle down in beaker and H2O is separated using decantation process. Observations: After placing the beaker over wire gauge and burning the Bunsen burner, the mixture of Cu(OH)2 in beaker becomes a little thicker. During the stirring, there were blue precipitates in the beaker floating in the mixture. After few minutes the mixture becomes dense and its color changes to brown and, then to black. This color change occurs slowly and gradually. After 5 minutes (approximately) the mixture starts boiling and there were no color change during boiling process. There were lots of bubbles and popped during boiling. After boiling for 1 minute the Bunsen burner is turned off.
- 3. After waiting for 5 or 6 minutes the black precipitates of CuO starts settling down leaving thewater up in the beaker. Before the boiling process, the volume of mixture was 100 mL but after the boiling it decreases to 75 mL. 3. Preparation of Chloride complex: Reagent HCL Appearance Clear and colorless Volume or Mass Concentration 30 Drops 6M liquid. The separated black colored precipitates of CuO is reacted with 6 M HCL solution. About 30 drops of 6 M HCL solution is added to CuO, so that every precipitate can dissolved to become chloride complex. Ionic Equation of formation of complex: Cu2+ (s) + 4CL-(aq) [CuCL4]2- Observations: After adding 10 drops of 6 M HCL into beaker containing CuO, the black precipitates of CuO changes to clear and dark green color. After 20 more drops of 6 M HCL, the color changes to yellow greenish chloride complex. While adding the drops there was repeatedly disappearance and appearance of black color.
- 4. 4. Preparation of ammonium complex. Reagent Appearance Volume or Mass Concentration NH3 Colorless solution 40 Drops 6M The yellow greenish chloride complex is reacted with NH4OH. The drops of NH4OH is added till we get a distinct color. Ionic Equation for the formation of complex: [CuCl4]2- (aq) + 4NH3 (aq) (in the presence of water) [Cu(NH3)4(H2O)2]2+(aq) + 4CL-(l) [Cu(NH3)4(H2O)2]2+(aq)+ 4H2O (l)(in equilibrium) 4NH3(aq) + [Cu(H2O)6]2+(aq) The most common complex in solution occurs is dark blue colored complex [Cu(H2O)6]2+ Observations: NH3OH solution gives pungent smell and it is clear and colorless solution. While adding the drops of NH3OH there was repeatedly disappearance and appearance of blue color. After adding 40 drops the whole complex solution changes to blue green color. While adding the NH3OH solution, white fumes come out rapidly and vigorously.
- 5. 5. Preparation of copper metal. Reagent Appearance Volume or Mass Concentration H2SO4 (aq) Clear and 15 mL 1.5 M Transparent Zn (s) (dust) Grey in color 0.15 g N/A Ethanol Clear and 5 mL N/A transparent solution The blue green ammonia complex is reacted with 15 mL of 1.5 M H2SO4 to make another complex, and then it is reacted to 0.15 g of Zn dust to obtain elemental Copper metal. The copper metal is then separated using vacuum filtration method. In this process, the copper metal is washed using 5 mL of 95% ethanol solution. [Cu(H2O)6]2+ (aq) + H2SO4(aq)Cu(SO4) (aq) +14H+(l) CuSO4 (aq) + Zn (s) Cu (s) + ZnSO4 (aq) Observation: After adding the 15 mL H2SO4 solution the color of complex changes to light blue and it becomes clear solution. After adding the 0.15 g of Zinc dust (grey color), the mixture complex starts bubbling vigorously and the precipitates start floating in the solution.
- 6. After continuous stirring, we get shiny brown color Cu metal from the mixture. The shape of Cu metal is distinct (clumps of Cu metal in solution) and can be clearly seen in the clear transparent solution. After vacuum filtration of Cu, we were left with shiny dark brown Cu metal of 0.0485g. Mass of Recovered Copper: Weighing bottle (empty) 11.1561 g Weighing bottle + Cu (s) 11.2046 g Mass of recovered Cu (s) 0.0485 g Theoretical value of copper: Molarity = 0.10 M Volume = 10 mL Into litres (volume = 0.01 L) No. of moles = Molarity * volume = 0.01 * 0.10 = 0.001 moles Yield = [(No. of moles * Molar mass of copper) / Molar mass of compound] = [(0.001 * 83.56 ) / 207.48]
- 7. = 0.0402 g Experimental value of copper = 0.0485 g Percent yield = [Mass of experimental yield/ Mass of theoretical yield] *100 = [0.0485 / 0.0402] * 100 = 120.6 % PART B In this part of experiment the compound of copper (I) ion will be formed using different reagents. There will be formation of two compounds in part B (a) CuCL and (b) Cu2O. 1. Preparation of CuCL. Reagent Appearance CuCl2.H2O Dark green color and cylindrical Volume or Mass Concentration N/A 0.30 g shape crystal. Ascorbic Acid White in color 0.30 g N/A CuCl compound is made by reacting the CuCL2.H2O with ascorbic acid, and then it is separated using vacuum filtration method.
- 8. Observations: The CuCL2.H2O crystals are heavy crystals. They are dark green in color and has cylindrical shape. The ascorbic acid is white inn color and is powdery. After adding 5 mL of distilled water into the powder and crystal mixture, the color of mixture changes to white but there is little greenish shade in mixture. The mixture becomes very dense after mixing with distilled water. After few minutes white precipitates settle down. During vacuum filtration of CuCL the color of powder changes into yellow color but there was still white powder in it. After 4 or 5 minutes its yellow color becomes brighter. After 5 more minutes it dries up but there was no specific color change and it remain yellow. 2. Preparation of Cu2O. Reagent Appearance Benedicts Reagent Clear, Dark blue color solution. Glucose Volume or Mass N/A 1 mL White and Clear solution Concentration 1% 1 mL
- 9. In this part, 1 mL of benedict reagent and 1 mL of glucose is taken into small test tube, and then it is placed in beaker containing water placed over the wire gauge and Bunsen burner is turned on to watch the color changes in the test tube. Observations: The benedict reagent has dark blue color and is clear, transparent solution. After adding the 1 mL of glucose to the benedict reagent the color of solution remain unchanged, that is, dark blue color. After turning on the Bunsen burner there were several color change in the test tube after few minutes. The upper layer in test tube changes in to yellow color, and then it color changes to brown color. This color change spread in the whole test tube rapidly. After some time, the upper layer of test tube changes to orange color and there wad brown color layer in the middle of test tube. The last layer of test tube was still dark blue colored. After 3 minutes the whole solution becomes brown red in color and it was very dense solution. During vacuum filtration of solution, there was no specific color change and it remains dark red brown in color.
- 10. Discussions: There are many sources of error in this experiment as we got more value of experimental copper (0.0485 g) than theoretical value of copper (0.0402 g). This experiment includes decantation process, which always produce error. Some precipitate is always lost when supernatant liquid is separated from the precipitate. Decantation errors could be avoided by employing filtration techniques instead of decantation technique. This experiment also includes the transfer of precipitate from a beaker to an evaporating dish. It is difficult to removeall of the precipitate from the beaker using simply liquid. Some precipitate sticks to the walls of the beaker and is extremely difficult to remove. The theory associated with this experiment in the atomic theory. The reason that the same mass of copper will be produced after all of the reactions that occur is because a constant number of copper molecules were present throughout the experiment. The original copper sample contained a specific number of moles of copper, and that amount of copper was present in every reaction, precipitate, and solution. Thus, the same number of moles of copper was produced in the final reaction because the amount of copper remained constant throughout the experiment. The atomic theory predicts this behavior.
- 11. Questions: 1. In the last step of Part A, we use Zn dust to react with mixture to obtain copper metal but if any Zn dust is still unreacted then it can affect the yield of copper metal. As Zn react with CuSO4 to give Cu and ZnSO4. If any zinc dust is not reacted then there will be CuSO4 in the mixture and we will not be able to get all yielding mass of copper metal in measurement. 2. In the experiment we observed the every reaction which leads to compound and complex formation. First we reacted the given solution of Cu(NO3)2 with NaOH to obtain copper hydroxide and it was blue in color, and then it is heated to get CuO. CuO is further used to form tow complexes (chloride and ammonia complex). The colors of complexes were identical. The copper chloride complex was yellow greenish in color and copper ammonia complex was blue green in color. Then we add concentrated H2SO4 and Zn dust to obtain shiny brown color copper metal. 3. Yes it is easy to distinguish between both oxidation sates of copper metal. Compound of copper (I) ion is not much stable as compared to copper (II) ions. Cu2+ ions form ionic compounds that have a range of solubility in water but the Cu+ ion is unstable in aqueous solution. Moreover, the copper is transition metal with different oxidation state but most stable state is copper (II). 4. The synthesis of copper metal is most relevant when it is done using various series of reactions and making complexes. After the complex formation of metal it can be extracted in more pure form. However, the extraction of metal can be done in one or two series of reactions but the metal could not be pure.
- 12. 5. The more value of experimental copper metal ( 0.0485 g) than theoretical value ( 0.0402 g) shows that there were an abundant errors in this lab. The error could occur while measuring over the analytical balance as a little disturbance over the shelf can vary its values. 6. This lab could be improved by using different technique of separating the precipitate instead of decantation process. Decantation can produce error so we should use some other filtration technique to obtain precipitates. Sources of Error: Throughout the entirety of the lab, there were many steps that presented possible sources of error such as adding too much reagent and less in copper solution, or it might be possible that precipitated are stuck with stirring rod, which can cause the error in reading. In step 2 decantation process is used to separate the precipitate which is biggest source of error as we can lose some amount of precipitate and some water can still be there in beaker which will react with reagent during the reaction of copper solution. The length of heating could also cause error as solution would need more 5 minutes to react completely with reagent. Last step of Part A require stirring that can also cause error as some of the copper metal can stuck with stirring rod. The precipitate of copper metal be left on the side of the beaker. While measuring the weight of copper metal the
- 13. balance may not calibrated properly. And for the first we are using the analytical balance which is accurate as 0.0004 g, the accuracy can be disturbed with small dust, sweat from our hand and also by the disturbance form the other students on the shelf it is placed on in the balance room. The error could also be occurred if all thedoors are not shut properly. The primary source of error is when we use vacuumfiltration process, the error could be produce because the pipes are not tightenedproperly or it is loose on the flask. Moreover some of the copper metal clumps can remain in the holes of flask. All in all, there was an abundance of error in this lab. Conclusion: This experiment was successful. The experimental value for copper metal obtained was 0.0485 g and theoretical value of copper was 0.0402 g.There were abundant of possibilities of error in this experiment. Familiarity with basic laboratory procedures was gained and working in group was plus point in this lab as we come to know more about the team work.
- 14. References: Lab manual: Koczanski, Krystyna; Xidos, James D. CHEM 1300 Laboratory Manual; UMSU Copy Centre: Winnipeg, MB, Canada, 2013, pp 16. Books with authors: Olmested, John III; Williams, Gerg; Burk, Robert C. Chemistry, Ist Canadian ed.; John Wiley and Sons Ltd: Mississauga, Canada, 2010, pp 399 – 406.
